The influence of mechano-active ion channels during kidney stone formation
Published in Bioengineering & Biotechnology, Chemistry, and General & Internal Medicine

In keeping up with my ongoing research-interest based posts about bottom-up bioengineering a pathological pathway of renal calcification; I initially addressed the concept relative to identifying a biological switch at the renal tip, with a follow-up post on the supporting role of machine learning and systems biology within the niche. In this new post, I take a different vantage point by combining structural biology and basic science, to gain deeper insights to translational bioengineering, by first focusing on regulating the pore-forming subunits Piezo1 and Piezo2 of mechano-active ion channels during progressive renal fibrosis and stone formation, with capacity to interfere the biological cascade for therapeutic intervention (Figure 1).
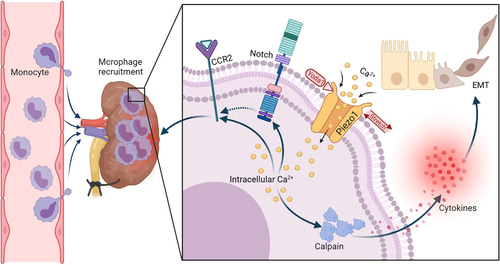
Figure 1: Piezo1 deletion in myeloid cells protects renal fibrosis by restraining macrophage infiltration and activation, a schematic representation [He 2022].
A tour-de-force discovery
Mechanotransduction is a key regulatory process underlying human health and disease and plays a primary role in the pathological pathway of renal fibrosis [Zhao 2022]. The process relies on special proteins known as mechanotransducers that convert mechanical forces to biochemical signals in cells. The capacity to sense physical forces is a conserved evolutionary trait present across all organisms, where cells convert mechanical stimuli into electrical or chemical signals via mechanically activated ion channels [Coste 2010, Kefauver 2020]. As with most discoveries, the proteins involved with mechanobiology were unknown at first, and biologists were armed only with a mechanosensitive channel signal that was detected about 40 years ago in 1984 [Guharay 1984].
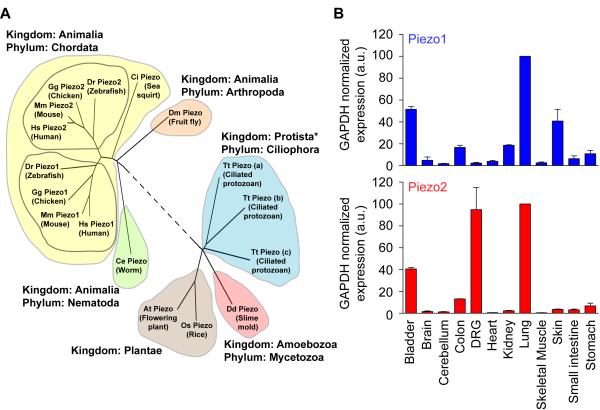
Figure 2: Evolutionary conservation and expression profile of mouse Piezo1 and Piezo2, completed by Patapoutian and team (Coste 2010).
The precise molecular identity of the mammalian proteins were revealed in 2010 by Ardem Patapoutian and colleagues, when the scientists carried out a groundbreaking ‘tour-de-force’ study, which included pressing cultured cells with a glass probe and simultaneously recording the whole-cell configuration through a patch clamp, to identify the evolutionarily conserved Piezo family of proteins (Figure 2), for which they subsequently received the Nobel prize in medicine and physiology in 2021 [Coste 2010, Kefauver 2020]. This discovery of receptors for ‘temperature and touch,’ revealed the role of Piezo proteins, so named from the Greek word ‘piesh’ meaning pressure, for the pressure-induced nature of the mechano-activated current. The team further demonstrated the capacity to mutate the Piezo1 gene and its homologous gene Piezo2 to alter the channel functions and gain insights to a variety of rare genetic diseases [Coste 2013].
The localized function of mechanosensory ion channels in renal pathology
The mechanically activated ion channels are highly expressed in endothelial cells, in the kidney, and the bladder. Functionally they are versatile, and broadly regulate the vascular structure, blood pressure homeostasis, osmoregulation, bone remodeling and osteogenesis, with impact on heart development, and in kidney disease (Figure 3) [Qin 2021, He 2022]. In the kidney, the Piezo1 proteins occur within the cortical and medullary collecting ducts, the proximal and convoluted tubule and renal corpuscle. In fibrotic kidneys, a higher expression of Piezo1 occurs in the human renal tubule and the main collecting duct [Zhao 2022], while influencing the expression of extracellular matrix proteins such as collagen type II and IX.
This in turn leads to exerting profibrotic effects, as experimentally observed in human kidney cell lines and with mouse proximal tubular cells [Fu 2021]. While both proteins form pore-forming subunits of mechanically activated channels to convert mechanical signals into biological cues to regulate physiological processes. The Piezo2 protein is predominantly present in sensory neurons, with specific expression in mesangial cells and in renin producing juxtaglomerular cells.
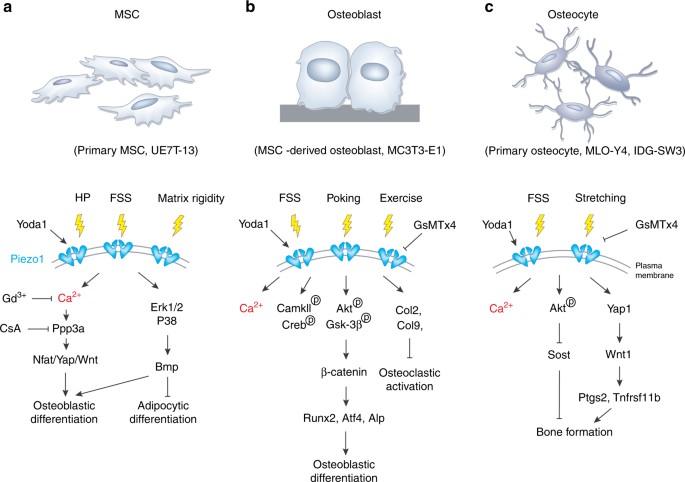
Figure 3: An example of Piezo1 signaling in the osteoblast lineage cells. (a) The activation of Piezo1 channels in primary mesenchymal stem cells can be triggered by hydrostatic pressure, fluid shear stress, matrix rigidity, and protein agonists to activate biological pathways via calcium signaling for osteoblast and adipocyte differentiation. (b) Mechanical stimuli such as fluid shear stress and poking can activate Piezo1 in mesangial stem-cell derived osteoblasts for differentiation. (c) Piezo1 channels from primary osteocytes can be triggered by fluid shear stress and stretching stimuli to activate Piezo1 and contribute to bone forming biological cascades [Qin 2021].
Both proteins play a significant role during sensory transduction during pressure-induced stress, to translate mechanical forces acting on the cell membrane into ion flux to initiate a bioelectric cascade that is understandable for the cells [Drobnik 2024]. In its general mechanism-of-action, an activated Piezo channel is permeable to Na+, Ca2+, K+, and Mg2+ ions, with a slight preference for calcium ions (Figure 4).
The extraordinary architecture of the mechanosensory ion channels
The Piezo proteins are activated by changes in membrane tension including shear-stress, stretch, compression, and osmotic stress [Coste 2012]. The subunits transduce internally and externally applied pressures across the cell membranes and sense the stiffness of the extracellular matrix. They are labelled as mechanotransducers with an unusually large protein composition of more than 2000 amino acids. The Yoda1 protein agonist is a powerful activator of the Piezo1 mechanosensory domain. These effects can be reversed with GsMTx4 antagonist – derived from a spider venom peptide [Gnanasambandam 2017]. Recent studies in 2018, showed new chemical activators of the Piezo1 channel known as Jedi1 and Jedi2, which exert their effects through different mechanisms to Yoda1, for mechanosensory ion channel activation [Wang 2018].
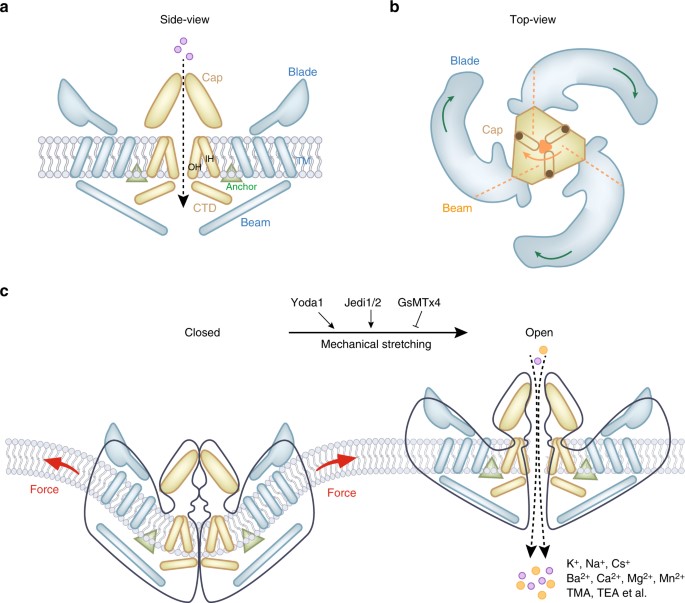
Figure 4: The mouse Piezo1 protein has a three-bladed propeller-shaped homotrimeric architecture. (a) Side view of mouse Piezo1 channel. (b) Top view of mouse Piezo1 channel. The three blades are assembled into functional trimers. (c) Mammalian Piezo1 proteins can be directly gated by membrane stretching, this is conserved throughout evolution. Yoda1 and Jedi1/2 are chemical activators of Piezo1 channels, and GsMTx4 is an antagonist of the Piezo1 channel. The channels are non-selective cationic mechanosensitive channels permeable to alkali ions, divalent cations, and several organic cations [Qin 2021].
The central pore of the channel is covered by a single cap with trimers around it, and are shaped like the blades of a propeller, and the structure is fundamental to mechanosensing and support (Figure 4) [Qin 2021]. The Piezo1 channels also function as blood flow sensors to align endothelial cells in response to blood flow. Loss-of-function experiments that knockdown Piezo1 in endothelial cells yield abnormal vessel development leading to atherosclerosis and cancer; pathologies that are commonly driven by alterations in shear stress and mechanical forces [Li 2014]. In fact, the global deletion of the Piezo1 gene is lethal to embryos, therefore it is conventional to generate a conditional knockout model of transgenic mice [Li 2014, Zhao 2016].
Parallels and contrasts: biomarkers and associated pathways of kidney ailments
Circling back to kidney fibrosis-related stone formation, no single theory has thus far provided a simple understanding of human kidney stone formation, due to their variety and complexity [Duffield 2014, Coe 2010]. In general, the origin of renal stone formation is attributed to deposits of microscopic plaque of calcium known as Randall’s plaque deposited in the interstitial tissue of renal papillae, to serve as a nidus for urinary stone formation [Chung 2014]. In 2010, Fedric Coe and colleagues described three independent pathways of human kidney stone formation, including free solution crystallization [Coe 2010]. Typically, renal tissue mineralization (nephrocalcinosis), stone formation (nephrolithiasis) and Randall’s plaque formation are distinct renal pathologies that contribute to renal calcification [Wiener 2018], accompanied with key biomarkers of calcification, renal fibrosis, oxidative stress, and epithelial-mesenchymal transitions [Arsenault 2016, Ho 2018].
In the spectrum of kidney ailments, fibrosis is characterized with myofibroblasts that appear within the interstitium of the kidney to synthesize collagen and is accompanied with cytoplasm contractile microfilament bundles composed of alpha smooth muscle actin (Figure 5) [Levey 2012, Duffield 2014]. The mechanosensitivity of Piezo proteins is based on the direct stimulation of cells or on the elasticity of the cellular environment. Changes across tissue stiffness dictate the pathological processes, for instance, the Young’s modulus of the healthy kidney parenchyma that is typically at 4 kPa can rise to 35 kPa with a stiff extracellular matrix due to tissue fibrosis in chronic kidney disease [Drobnik 2024]. Mechanical forces can physically impact cells by regulating their morphology and orchestrate the interplay between chemical and mechanical cues to influence cell fate during regular development vs. pathology [Mammoto 2010].
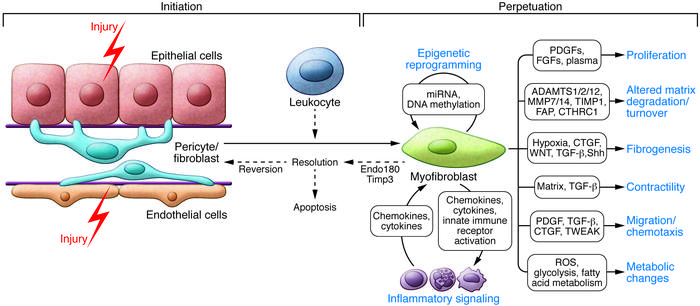
Figure 5: Cellular and molecular mechanisms in kidney fibrosis. Shown is the initiation of the fibrogenic process, mechanisms that perpetuate the fibrogenic state and consequences of myofibroblast persistence to regulate the resulting inflammatory response [Duffield 2014].
When compared to chronic kidney disease, the etiology of renal stone formation is not clearly established [Coe 2010], as a result, the process of bottom-up engineering a pathological pathway of renal stone formation is a work-in-progress that relies on construing experimental evidence using tissue biopsies and intraoperative imaging [Coe 2010, Zhao 2022]. Renal fibrosis - a pathological biomarker of chronic kidney disease is also similarly observed in stone formers; therefore, the pathway can equally rely on stretch and compression-related mechano-activation of Piezo channels to increase the surrounding tissue stiffness towards renal calcification [Drobnik 2024].
Transforming growth factor β1 is another key cytokine upregulated via Piezo1 activation to support the development of renal fibrosis by elevating the extracellular matrix deposition (Figure 5). The cytokine upregulates the synthesis of proteins such as collagen, while inducing epithelial-mesenchymal transitions that are also established biomarkers in the renal papillae of stone formers [Duffield 2014, Gu 2020]. Furthermore, the transcriptomics of the renal papillae of stone forming patients, have revealed the incidence of a biological switch activated oxidative stress pathway in renal tubular cells at the renal tip [Han 2013], an aspect that I briefly covered in an earlier post.
Attention to detail: the profibrotic Piezo1 calcium-calpain2-integrin β1-fibronectin pathway.
During the pressure-activated downstream cascade of renal fibrosis, the protein Yoda1 is a powerful trigger and agonist of the upstream element Piezo1 [Botello-Smith 2019, Lacroix 2018]. The profibrotic factors of the biological cascade are listed in table 1. The process upregulates fibronectin, transforming growth factor β1 and collagen [Zhao 2022]; biomarkers that are likewise observed in the renal papillae of stone forming patients [Khan 2012]. In its mechanism-of-action, the activated Piezo channels increase the intracellular calcium influx in human kidney cells to activate calpain2; a calcium-dependent protease that initiates the profibrotic response [Drobnik 2024, Xu 2023].
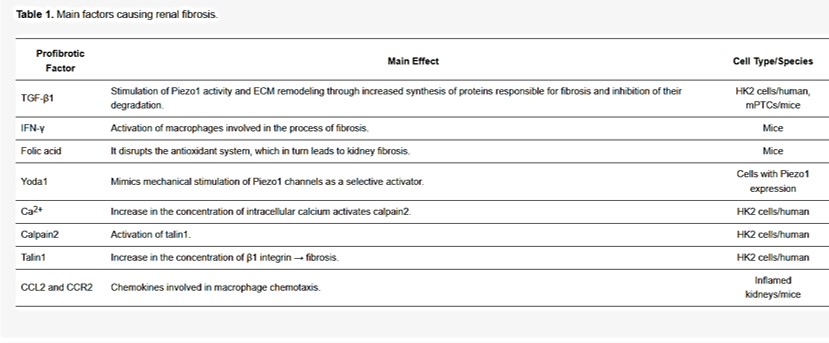
Table 1: Profibrotic factors underlying renal fibrosis [Drobnik 2024].
In a nutshell, the biological cascade begins with Piezo1 activation by mechanical stimulus in the presence of Yoda1, which activates calpain2, which then increases the amount of talin1 by cleaving the compound; a constituent adhesion complex protein for cell-matrix adhesion that ultimately promotes increased deposits of extracellular matrix for tissue fibrosis, resulting in a notably increased Young’s moduli of the surrounding tissue [Drobnik 2024, Klapholz 2017]. The feed-forward mechanism of increased intracellular calcium influx can continue to stimulate calpain2, further activating talin1, and integrin β1 for continuous extracellular matrix deposition for a profibrotic outcome (Figure 6) [Fu 2021].
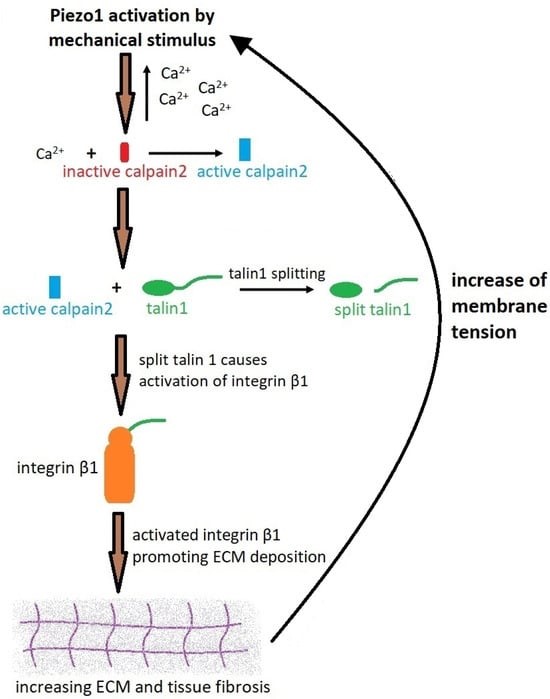
Figure 6: A schematic of Piezo1 activation and the process of fibrosis via the calcium-calpain2-integrin β1-fibronectin pathway [Drobnik 2024].
Studies performed in calpain2 knockdown kidney cells have already shown a significant decrease in fibronectin and alpha smooth muscle actin levels post-treatment, even in the presence of the agonist Yoda1 [Zhao 2022]. The Piezo1 initiated calcium-calpain2-integrin β1-fibronectin pathway is therefore a notable pathological cascade for therapeutic intervention during kidney fibrosis (Figure 7).
Early outcomes: experimental validation of stress-initiated pathological mechanisms.
Research on Piezo1 in the kidney is thus far in its infancy. In vitro experiments conducted within renal mesangial cell environments under specific conditions can aggravate the fibrotic process for increased extracellular matrix deposition [Fu 2021]. The pathological process increased the translocation of the transcription factor Yes-associated protein (YAP) to the cell nucleus; an effect that depends on the p38 MAPK pathway; well-known for its evolutionarily conserved role in transducing stress signals from the environment [Fu 2021, Drobnik 2024].
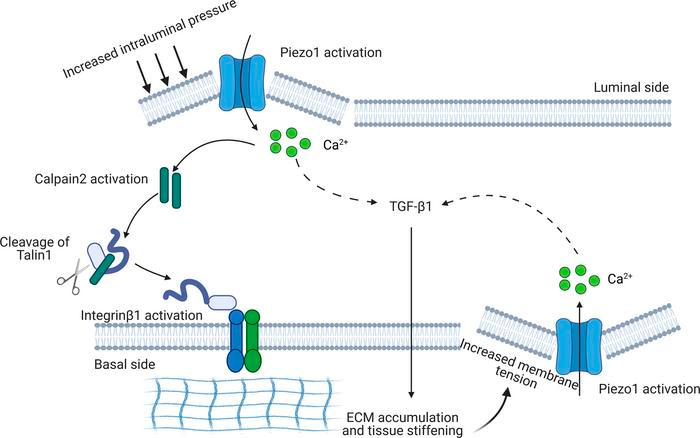
Figure 7: Reiterating the biological cascade shown in Figure 6., in a fibrotic kidney, a potential reciprocal Piezo1-dependent feedforward mechanism in kidney fibrosis [Fu 2021].
Translational studies have also highlighted the significance of the Piezo activated profibrotic pathway in a preclinical mouse model, often recreated as a model of unilateral ureteral obstruction (UUO) or via folic acid induction, to cause kidney fibrosis by aggregating the Piezo1 protein [Zhao 2022]. Researchers have also used the short hairpin RNA to target Piezo1 and lower its expression in animal models of the disease, for an outcome of reduced fibrosis and improved kidney function by altering the Piezo1-YAP-p38 MAPK pathway [Fu 2021]. This can be further elaborated via transcriptome studies in proximal tubule cell culture models [Khundmiri 2021] and with cell-specific deletion of Piezo1 in mice, to inhibit macrophage infiltration, and protect mice against renal fibrosis [He 2022].
Identifying the culprits and their mechanisms for therapeutic intervention
As mentioned in an earlier post, the key to disease-oriented basic science research is based on analyzing the culprit proteins, to discover druggable targets as a blueprint for drug discovery and development. Among the pharmacological inhibitors and stimulators of piezo channels, streptomycin, and an amphipathic spider peptide toxin GsMTx4 are potent inhibitors of Piezo1 currents that can reverse the effects of renal fibrosis that are triggered via Piezo1 [Drobnik 2024]. While the effect of Piezo1 inhibition is reversible, these inhibitors are not selective for the Piezo1 channel alone, with general capacity to block cationic mechanosensitive channels [Gnanasambandam 2017].
Products of fibrosis-related pathways such as increased calpain2 too can be ameliorated by the GsMTx4 spider peptide toxin, to prevent renal fibrosis in animal models of unilateral ureteral obstruction (Figure 8). Zhao and colleagues conducted a series of elegant experiments to show how the effect of GsMTx4 or siRNA silencing of Piezo1 proteins can completely suppress mechanical stress induced intracellular calcium enhancements [Zhao 2022].
Moreover, biologists have highlighted the relationship between Piezo1 activated macrophages during renal fibrosis in fibrotic kidneys via fluorescence colocalization [He 2022]. Furthermore, when Piezo channels were genetically engineered by the knockdown of Piezo1 gene in myeloid cells, it protected the mouse model from fibrosis, to emphasize its indispensable nature for renal pathology. Gathering deeper insights to structural moieties and protein associations in renal fibrosis can provide further evidence to develop timely therapeutic interventions.
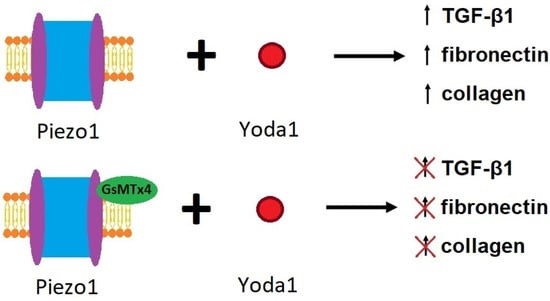
Figure 8: The effects of Yoda1 and GsMTx4 on Piezo1 and the process of fibrosis [Zhao 2022].
Mechanobiology-on-a-chip for precision diagnostics and precision medicine
The Piezo1 protein is sensitive to mechanical stimuli, so the ion channel can mediate compression and stretch to induce fibrosis; a trait that can be explored in the lab [Zhao 2022]. Beyond the practical use of animal models, pathological mechanisms can be recreated with human kidney cell lines for a more accurate picture of the local environment during kidney fibrosis, by inducing mechanical shear compression and stretch in a microphysiological organ-chip environment in vitro [Kaarj 2019].
The microfluidic platform is ideal for Piezo1 to sense microenvironmental stiffness, and to transduce external mechanical stimuli into intracellular signaling pathways, to observe increased levels of Piezo1 mRNA and immunofluorescence, alongside the upregulation of biomarkers such as smooth muscle actin and fibronectin [Lacroix 2018, Ergir 2018]. Renal fibrosis triggered on a chip of 3D cultured cells derived from renal stone forming patients can be partially or fully inhibited in the in vitro model, by using new or existing therapeutic agents, such as GsMTx4, or by culturing genetically engineered cells that have undergone siRNA silencing of Piezo1 to explore the role of mechanotransduction during renal stone formation [Ergir 2018, Xu 2023].
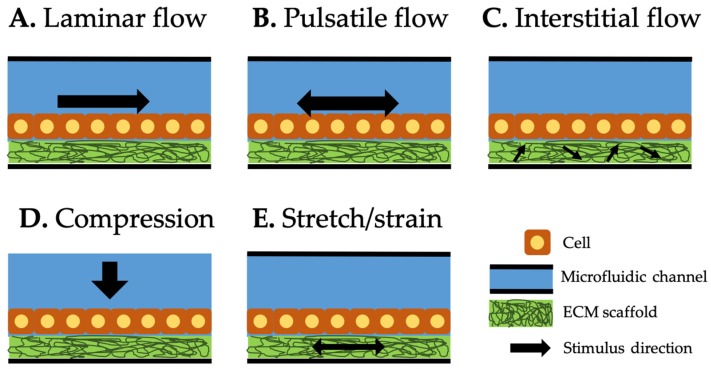
Figure 9: Type of mechanical stimuli integrated in organ-on-a-chip systems. Black arrow represents the direction of each stimulus. Laminar (A) and pulsatile (B) flows along the microfluidic channel. Interstitial flow (C) through the extracellular matrix scaffold generates shear stress on the attached cells. Compression (D) generates compressive force on top of the cells. Stretch/strain (E) generates the force to the cells attached to it [Kaarj 2019].
Mechanical stimuli integrated and induced on a chip (mechanobiology-on-a-chip) is classified into three categories: shear flow, compression, and stretch/strain [Kaarj 2019]. During its mechanisms-of-action, the laminar or pulsatile flows along the microfluidic channel can trigger the Piezo channels on cell surfaces. The concept of interstitial flow can act within the extracellular matrix scaffold to generate shear stress on attached cells. Compression can be generated as a compressive force on top of cells for Piezo activation and stretch/strain forces can be separately applied to the extracellular matrix to generate the force of cell attachment, to transduce the pathological pathways in a microphysiological environment (Figure 9).
An afterthought – bridging the in vivo and in vitro gap in mechanobiology in renal fibrosis.
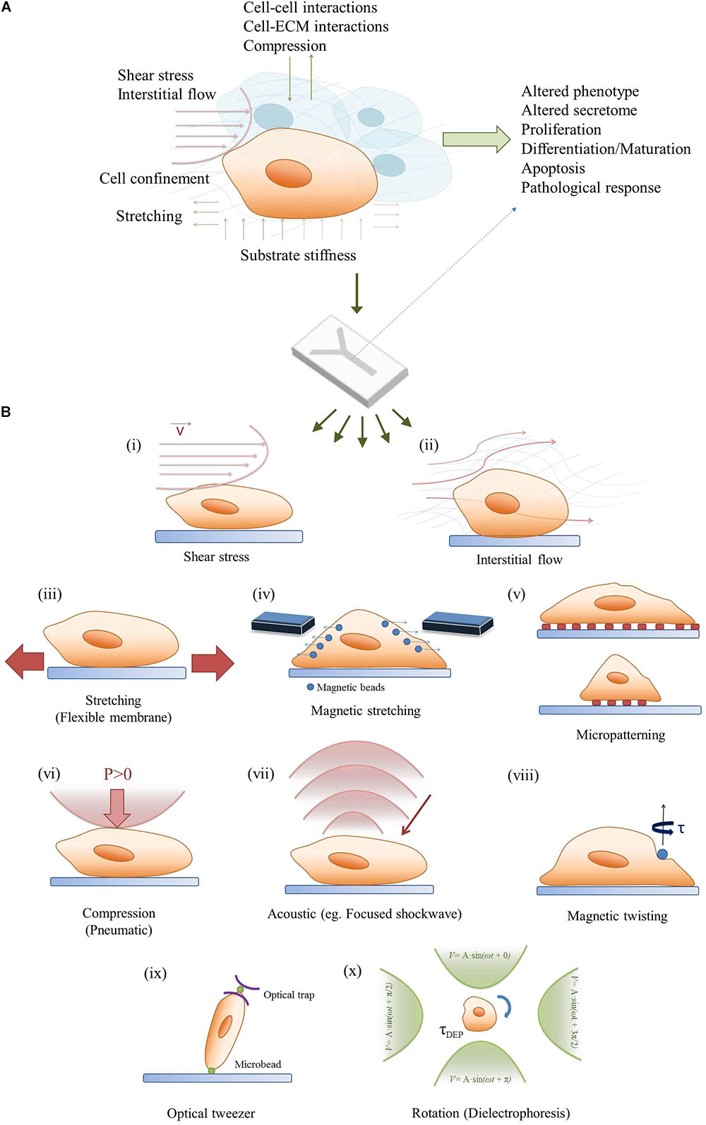
Figure 10: Bridging the in vivo/in vitro gap of mechanobiology a) combining mechanobiology cues in the microenvironment to regulate cell signaling, b) a simplified demonstration of the methods of regulating mechanobiology-on-a-chip with cell cultures [Ergir 2018].
The pathology of Piezo activated renal fibrosis in renal disease progression is a well-documented mechanobiological cascade that highlights the mechanosensory and mechanotransduction capacity of renal epithelial cells to aggravate the phenomenon [Ergir 2018]. In comparison to chronic kidney disease, the etiology of stone formation is not clearly established, albeit with the observation of analogous biomarkers of renal fibrosis [Coe 2010, Khan 2012]. Bottom-up engineering a pathological pathway of renal stone formation is therefore a work in progress, supplemented with tissue biopsies of the renal papillary tip region for cell culture and histopathology, alongside intraoperative imaging [Coe 2010, Zhao 2022]. Transcriptomics derived from the renal tissue of stone-forming patients, and mechanobiology-on-a-chip experiments conducted with renal cells and biopsies, offer a wealth of information to explore the underlying physico-chemical output, to regulate cell signaling and the patient tissue response. To ask and answer key questions of disease-oriented basic science during renal stone formation.
- Is there a biological switch at the renal tip that triggers the onset of renal calcification?
- Does Piezo-activated mechanotransduction induce the upregulation of renal fibrosis during calcification and contribute to kidney stone formation?
- Can identifying the culprit proteins and regulatory biological mechanisms, offer a potential target of pharmacotherapy in attenuating kidney disease?
Since research on Piezo1 in the kidney is still in its infancy [Zhao 2022], it is plausible to combine next-generation microfluidic strategies within the context of translational bioengineering to recreate microphysiological environments and explore complex disease mechanisms within advanced in vitro models to answer these key questions. Progressive experiments can build the answers to bridge the in vivo and in vitro gap of understanding the pathology of renal calcification.
Header Image: A technical note on the proximal tubule kidney chip for modeling human physiology, Emulate.
References
- He Y. et al. Myeloid Piezo1 Deletion Protects Renal Fibrosis by Restraining Macrophage Infiltration and Activation, Hypertension, 2022.
- Zhao X. et al. Mechanosensitive Piezo1 channels mediate renal fibrosis, JCI Insight, 2022.
- Coste B. et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels, Science, 2010.
- Kefauver J.M. et al. Discoveries in structure and physiology of mechanically activated ion channels, Nature, 2020.
- Guharay F. et al. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle, The Journal of Physiology, 1984.
- Coste B. et al. Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of Distal Arthrogryposis, PNAS, 2013.
- Qin L. et al. Roles of mechanosensitive channel Piezo1/2 proteins in skeleton and other tissues, Bone Research, 2021.
- Fu Y. et al. Targeting Mechanosensitive Piezo1 Alleviated Renal Fibrosis Through p38MAPK-YAP Pathway, Frontiers Developmental Cell Biology, 2021.
- Drobnik M. et al. Mechanosensitive Cation Channel Piezo1 Is Involved in Renal Fibrosis Induction, MDPI Molecular Sciences, 2024.
- Coste B. et al. Piezo proteins are pore-forming subunits of mechanically activated channels, Nature, 2012.
- Gnanasambandam R. et al. GsMTx4: Mechanism of Inhibiting Mechanosensitive Ion Channels, Biophysical Journal, 2017.
- Wang Y. et al. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel, Nature Communications, 2018.
- Li J. et al. Piezo1 integration of vascular architecture with physiological force, Nature, 2014.
- Zhao Q. et al. Ion Permeation and Mechanotransduction Mechanisms of Mechanosensitive Piezo Channels, Neuron, 2016.
- Duffield J. S., Cellular and molecular mechanisms in kidney fibrosis, JCI, 2014.
- Coe F. et al. Three pathways for human kidney stone formation, Springer Link, 2010.
- Chung H. et al. The role of Randall plaques on kidney stone formation, Translational Andrology and Urology, 2014.
- Wiener S. et al. Novel insights into renal mineralization and stone formation through advanced imaging modalities, Connective Tissue Research, 2018.
- Arsenault P. et al. The Zinc Finger of Prolyl Hydroxylase Domain Protein 2 Is Essential for Efficient Hydroxylation of Hypoxia-Inducible Factor α, Molecular and Cell Biology, 2016.
- Ho S. et al. Architecture-Guided Fluid Flow Directs Renal Biomineralization, Scientific Reports, 2018.
- Levey A. et al. Chronic kidney disease, Lancet, 2012.
- Mammoto T., and Ingber D. et al. Mechanical control of tissue and organ development, Development, 2010.
- Gu Y. et al. Diverse Role of TGF-β in Kidney Disease, Frontiers Cell and Developmental Biology, 2020.
- Han W. et al. Hypoxia-inducible factor prolyl-hydroxylase-2 mediates transforming growth factor beta 1-induced epithelial–mesenchymal transition in renal tubular cells, BBA, 2013.
- Botello-Smith W. et al. A mechanism for the activation of the mechanosensitive Piezo1 channel by the small molecule Yoda1, Nature Communications, 2019.
- Lacroix J. et al. Probing the gating mechanism of the mechanosensitive channel Piezo1 with the small molecule Yoda1, Nature Communications, 2018.
- Khan S. et al. Association of Randall's Plaques with Collagen Fibers and Membrane Vesicles, Journal of Urology, 2012.
- Xu Y. et al. Mechanotransductive receptor Piezo1 as a promising target in the treatment of fibrosis diseases, Frontiers Molecular Bioscience, 2023.
- Klapholz B. et al. Talin – the master of integrin adhesions, Journal of Cell Science, 2017.
- Khundmiri S. et al. Transcriptomes of Major Proximal Tubule Cell Culture Models, JASN, 2021.
- Kaarj K. et al. Methods of Delivering Mechanical Stimuli to Organ-on-a-Chip, Micromachines, 2019.
- Ergir E. et al. Small Force, Big Impact: Next Generation Organ-on-a-Chip Systems Incorporating Biomechanical Cues, Frontiers Physiology, 2018.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in