One of the main barriers to a cure of AIDS is the persistence of HIV in a small subset of long-living resting CD4⁺ T cells in a transcriptionally silent form. Although combined antiretroviral therapy (cART) efficiently suppresses viral replication and prevents development of AIDS, it does not eliminate these latent viral reservoirs. Upon therapy interruption, HIV reactivates in most people living with HIV (PLWH) within a few weeks, causing spreading infection and disease. Thus, treatment must currently be maintained for life and eradication of the latent viral reservoirs has become a top priority in AIDS research.
To address this problem, the “shock and kill” strategy has been proposed. In this approach, latency reversing agents (LRAs) are used to awaken dormant proviruses in patients on cART. Consequently, the infected cells start producing virions and become “visible” to the immune system and susceptible to clearance. However, current LRAs fail to significantly reduce the frequencies of latently infected cells and often cause severe side effects. Thus, novel safe and efficient LRAs are urgently needed to improve cure strategies.
One important question is which endogenous circulating factors control the establishment and reactivation of the viral reservoir in vivo. Identifying endogenous LRAs may expand our therapeutic possibilities by “waking up” dormant HIV proviruses and shed light into natural mechanisms controlling latency.
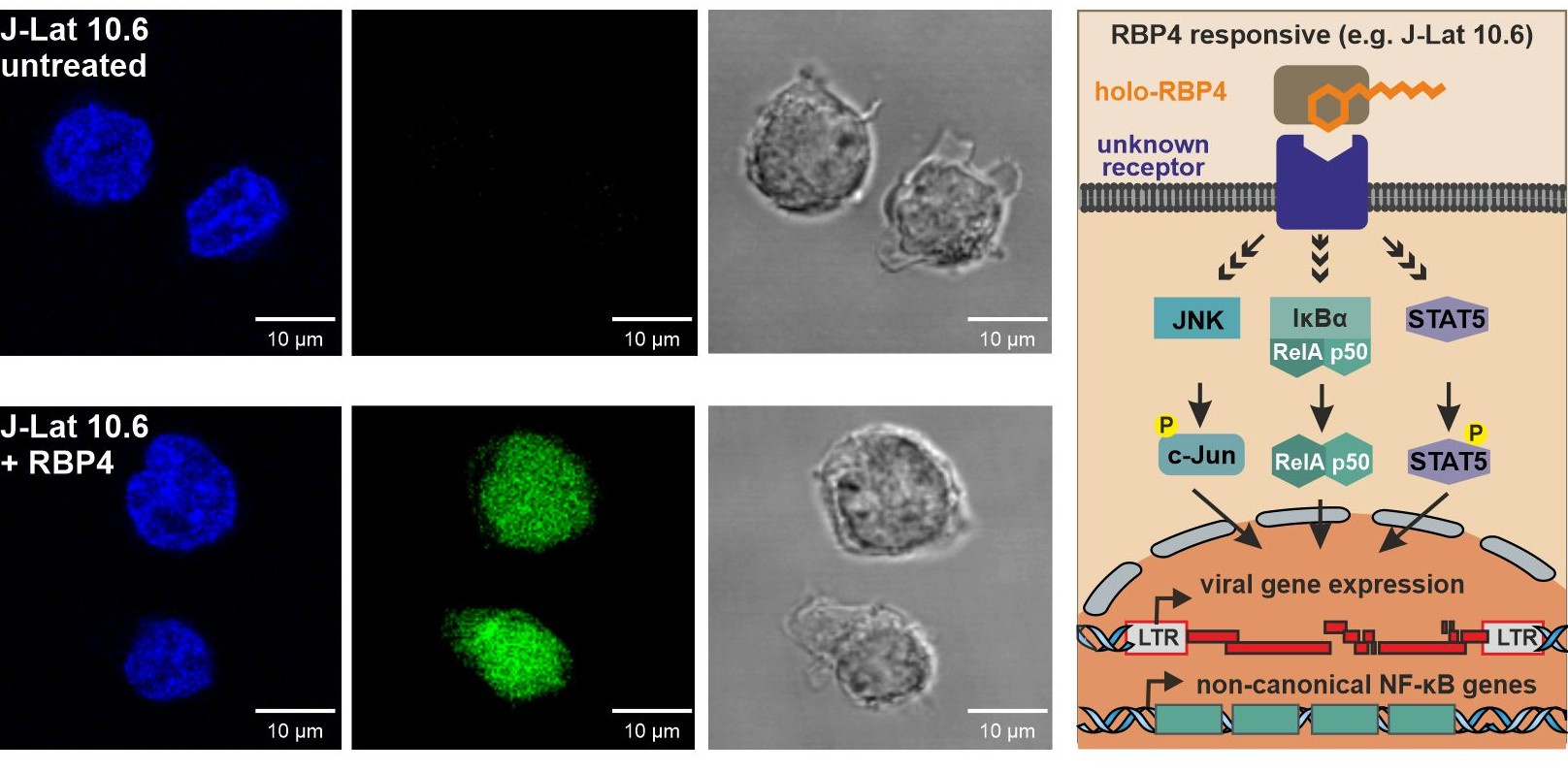
In our interdisciplinary study, we examined human blood for natural compounds capable of reactivating hidden HIV. To achieve this, we screened a human hemofiltrate-derived peptide library that encompasses essentially all peptides and small proteins circulating in blood. We discovered that Retinol Binding Protein 4 (RBP4), the primary transporter of vitamin A (retinol) in the bloodstream, acts as a potent endogenous HIV-1 latency reversing agent. RBP4 strongly activates HIV transcription in various subclones of Jurkat T cells harbouring transcriptionally silent HIV-1 proviruses carrying a GFP reporter. While many LRAs show promising activity in vitro, they often fail to significantly reduce latent HIV-1 reservoirs in clinical trials. Notably, collaborative work with the group of Luis Montaner demonstrated that RBP4 induces HIV-1 activation in a significant fraction of primary T cells isolated from infected individuals under effective cART and with undetectable viral loads, offering prospects for a relevant role in vivo.
RBP4 circulates in two isoforms: holo-RBP4 (retinol-bound) and apo-RBP4 (retinol-free). In the bloodstream, holo-RBP4 associates with transthyretin (TTR) to prevent renal clearance. Upon retinol delivery, RBP4 shifts to the apo form, which is subsequently filtered through the kidneys and excreted. This raised a central question: is the retinol required for RBP4-mediated HIV latency reversal activity? To answer this, we joined forces with the research group of Frank Rosenau and found that only holo-RBP4 reactivates HIV, while free forms of retinoids, such as retinol, retinal and retinoic acid are inactive. Furthermore, removal of retinol from circulating holo-RBP4 abolished its activity as LRA. Thus, the physiological retinol-RBP4 complex seems essential for reactivation of latent HIV proviruses, yet this effect is independent on retinol delivery and its conversion to retinoic acid.
If RBP4 reactivates HIV without releasing vitamin A into target cells - how does it act? Latently infected T cell lines differed strongly in their responsiveness to RBP4. To obtain insights into the underlying mechanisms, we thus analysed the transcriptional responses to RBP4 in different J-Lat subclones. Indeed, transcriptomic analyses revealed that RBP4 selectively induces a surprisingly small set of cellular genes linked to the non-canonical NF-κB pathway in responsive cells. These host genes are likely regulated by the canonical NF-κB transcription factor RELA. Previous studies support a cooperative role of JAK/STAT, JNK and NF-κB pathways in HIV-1 reactivation in the J-Lat system. Guided by this, we performed comprehensive genetic knockouts and pharmacological inhibition studies of factors involved in the mentioned signalling pathways. Together, these cooperative studies revealed that the canonical NF-κB pathway drives the initial RBP4-mediated HIV reactivation but JAK/STAT5 and JNK signalling contribute to efficient and sustained responses.
An open question is whether responsiveness is associated with differential expression of a holo-RBP4-specific receptor. STRA6, the main receptor for RBP4, and TLR4, previously implicated in RBP4-induced immune signalling, were the main candidates. However, our results showed that STRA6 is expressed at similar levels in both RBP4 responsive and non-responsive J-Lat subclones, and TLR4 was absent altogether. Thus, one future goal is to identify the potential as-yet-unknown receptor that allows holo-RBP4 to activate latent HIV in CD4⁺ T cells.
Altogether, our unexpected discoveries identify holo-RBP4 as effective natural activator of dormant HIV proviruses that functions at physiological concentrations in both cell lines and patient-derived primary cells. Notably, this discovery not only expands our understanding of how endogenous factors may regulate HIV reservoirs and offers new directions for therapeutic strategies, but also provides a proof-of-concept that systematic screening of peptide libraries from body fluids can reveal novel natural LRAs. However, important questions remain to be answered. What is the unknown receptor that mediates holo-RBP4 activity? Could RBP4 work synergistically with other circulating factors to shape the viral reservoir in vivo? It is known that HIV-1 latency involves various cellular features and epigenetic mechanisms, including histone modifications and host silencing factors. Thus, does differential responsiveness of latently infected cells to RBP4 reflect differences in receptor expression or are epigenetic constraints at proviral integration sites equally important? We are currently addressing these questions and applying our approach to identify additional endogenous factors capable of reverting latency in models that are poorly responsive to known LRAs. We are confident that these efforts may uncover novel physiological pathways controlling HIV persistence and open new avenues for cure strategies.
Follow the Topic
-
Signal Transduction and Targeted Therapy

This is an international, peer-reviewed, open-access journal publishing articles related to signal transduction in physiological and pathological processes, alongside signal transduction-targeted therapeutics in the form of biological agents and small molecular drugs used to treat human diseases.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in