Thermal significance of hidden colors: Insights from the evolutionary history of vipers
Published in Ecology & Evolution

Animals are covered head-to-tail in colors, some of which are used in functions as diverse as crypsis, communication, and thermoregulation. The typical locomotory method of snakes (i.e. crawling on the ground) renders their ventral (belly) colors seemingly superfluous. What significance could a color that is out of sight and typically pressed again the substrate have? To understand, it is useful to think about the relationship between colors and heat.
Imagine you arrive at a nice white-sand beach in the morning and enjoy the breeze and sound of waves while laying down on your towel. Around 12 pm you get hungry, and walk to get a sandwich at a close-by café. A few seconds later, you realize that, unlike a few hours ago, the sand is hot, and you run back to your towel to put on your flip flops. Well, this is due to one of the thermal properties of materials, known as specific heat capacity. Every material absorbs and release heat, but this proportion can significantly differ between substances. Dry sand has a low specific heat capacity, meaning that it takes less energy to increase its temperature compared to other water-rich substrates.
All things being equal, a bright material such as the white sand, absorbs less solar radiation than a dark material. This property is also applicable to the integument. Thanks to a class of multifunctional macromolecules called melanins, the pigmented integument can selectively absorb and reflect solar and environmental radiation, ultimately affecting the body temperature of organisms (Fig. 1), causing darker individuals to heat up faster.
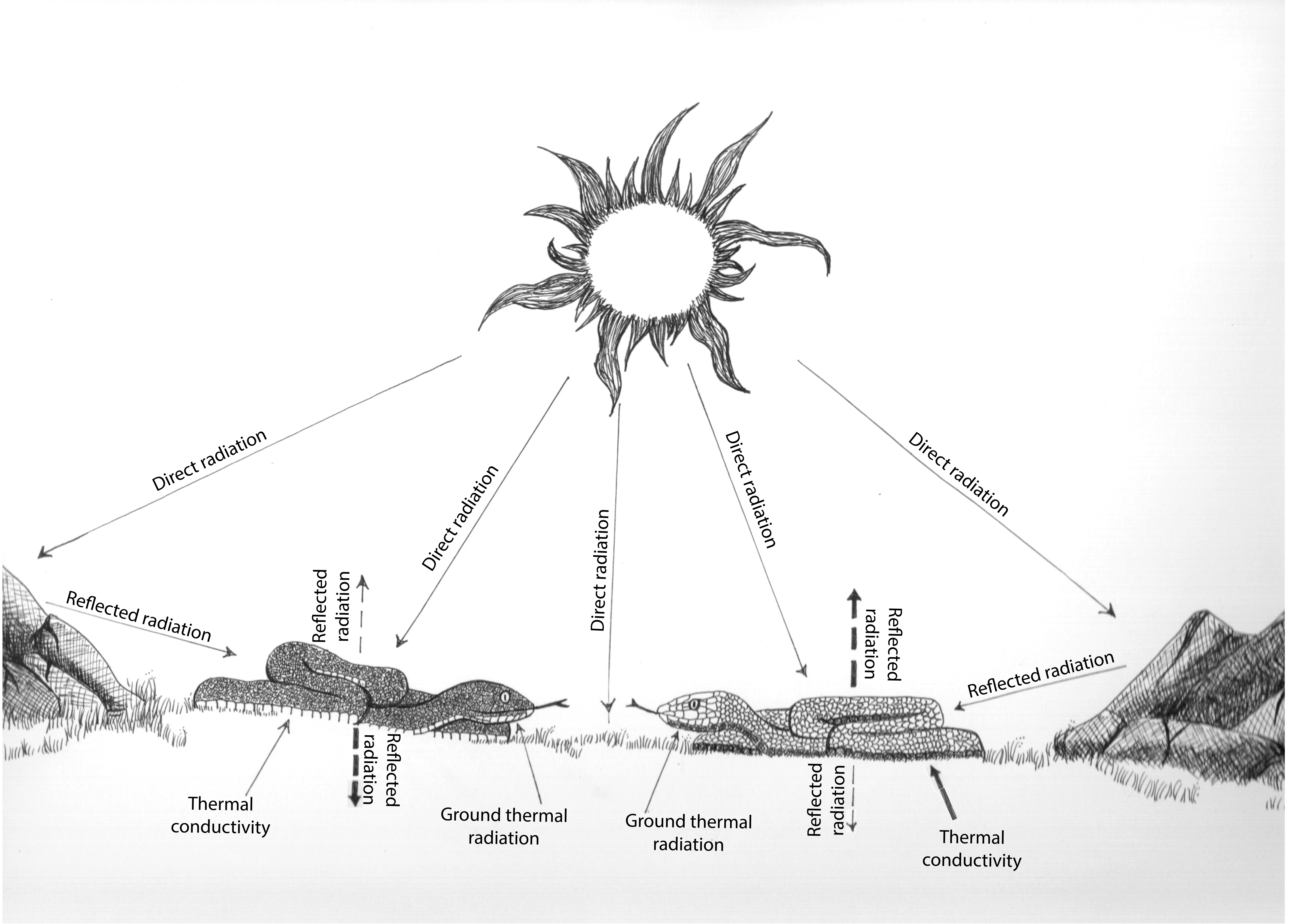
We therefore wondered what happens to an animal when it lives in close contact with a hot substrate. How does it cope? By behavioral adaptations? Or perhaps the integument itself can help dissipate the heat?
Squamate coloration has long been investigated, but much less attention has been paid to their ventral coloration, where social-and-sexual selection is clearly not in play.
In our recent study we aimed to fill the gap in this field. By using vipers as study organisms (Fig. 2), we took an eco-evolutionary approach to understand what drives the variation of color brightness in different body regions, including the cryptic ventral region.

But then came the hard part. How do we obtain the integument brightness of vipers? Obtaining spectrophotometric measures from living vipers would be... inadvisable, and colors of alcohol-maintained museum specimens fade. This leaves image analysis as an option but, unlike birds, no single unified pictorial guide to vipers exists, and we were thus left with unstandardized images from the web.
We thus developed a macro in ImageJ that enables users to select the body region of interest and automatically calculates RGB values and converts them to brightness levels. And what about the lack of image standardization? We accounted for different lighting and setup conditions using several precautionary steps. We selected multiple pictures per body region per species, assessed their variability, avoided over/under exposed areas, and most importantly, we selected 29 living reptiles (Fig. 3) to assess the relationship between brightness data obtained from image analyses and spectrophotometry and to verify the relationship between the visible, near infrared and ultraviolet spectra. All together, we showed that following this approach we can obtain reliable estimations of species integument brightness via image analyses. We ultimately analyzed the brightness of 4161 publicly available images from 126 species.

Our results show that substrate type influences the evolution of ventral brightness, where low specific heat capacity substrates (hot substrates) strongly associate with bright ventral colors, likely for efficient heat transfer. The results suggest that following the aridification phase Earth experienced during the Miocene (~ 23-5 Ma), vipers were able to exploit new arid vacant niches partly due to the enhancement of bright ventral colors.
With this research we provided evidence for the significance of brightness of an often-neglected body region and we suggest implementing ventral reflectance in climate change risk assessments due to the potential far-reaching impact it can have on species’ ecology.
References
Goldenberg, J., D’Alba, L., Bisschop, K., Vanthournout, B., & Shawkey, M. (2021). Substrate thermal properties influence ventral brightness evolution in ectotherms. Communications Biology, 4, 26. https://doi.org/10.1038/s42003-020-01524-w
Porter, W. P., & Gates, D. M. (1969). Thermodynamic equilibria of animals with environment. Ecological monographs, 39(2), 227-244. https://doi.org/10.2307/1948545
Porter, W. P., Mitchell, J. W., Beckman, W. A., & DeWitt, C. B. (1973). Behavioral implications of mechanistic ecology - Thermal and behavioral modeling of desert ectotherms and their microenvironment. Oecologia, 13(1), 1-54. https://doi.org/10.1007/
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in