Timing Matters: Disease Progression Turns Innocuous Nanoparticles Into Inflammatory Agents
Published in Materials and Immunology
When it comes to drug delivery, various strategies are used to administer therapeutics, ranging from tablets to intravenous injections and more. Each strategy has their own benefits, and one promising approach involves loading drugs within nanoparticle formulations. These nano-sized, delivery vehicles are synthesized from polymers, lipids, proteins, or metals, and designed to prolong drug circulation, reduce off-target toxicity, and enhance delivery to specific cell types. Over the years, nanoparticles have been applied clinically in a wide range of applications, such as cancer, infection, imaging, and mRNA vaccines. However, despite their potential, the clinical translation of nanoparticle therapies has been limited. This can be attributed, in-part, to diminished therapeutic efficacy and high inter-patient variability often presenting more variably than the soluble drugs themselves, stifling clinical translation.
This raised an important question for our team: How does disease progression and the timing of nanoparticle administration influence their interactions with the immune system? Could this be a contributor to the observed clinical variability?
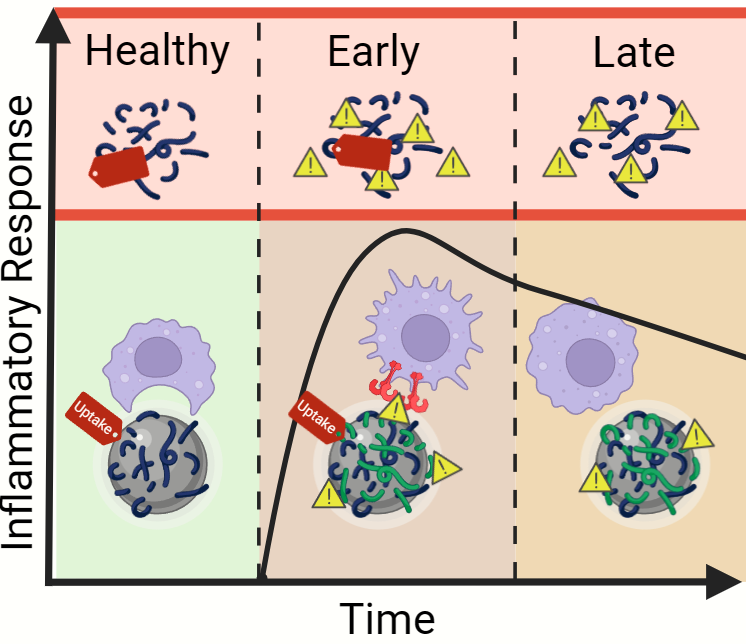
When nanoparticles enter biological matrices, like blood, they are rapidly coated by endogenous biomolecules forming a “biomolecular corona.” The corona is made up of proteins, lipids, and other molecules, and its composition can significantly influence nanobio interactions and the recognition of nanoparticles by the immune system, leading to enhanced immune clearance, impaired targeting, and reduced therapeutic efficacy. It is well-known that the composition of the corona is highly dependent on the patient’s blood composition, termed the “personalized biomolecular corona.” Since the corona composition can be highly influenced by the host’s disease state, we hypothesized that disease dynamics would reshape the nanoparticle corona, altering immune recognition, and induce unintended immune activation.
To explore this question, we used a mouse model of sterile systemic inflammation. By intraperitoneally injecting lipopolysaccharide (LPS), a component of Gram-negative bacteria, a controlled systemic inflammatory response was induced in vivo. This model allowed us to reproducibly examine different stages of inflammatory disease progression to assess how systemic inflammation alters plasma biomolecule dynamics and subsequent nanoparticle corona-dependent biological responses. We used polymeric nanoparticles made from poly(lactic-co-glycolic acid) (PLGA) as a representative formulation given its prevalent pre-clinical use. Our findings reveal that the dynamics of the inflammatory response significantly changes the composition of the nanoparticle corona, which in turn affects how immune cells interact with nanoparticles. Specifically, during early stages of inflammation, nanoparticles sequester pro-inflammatory biomolecules from the plasma, transforming previously inert nanoparticles into inflammatory agents that induce pro-inflammatory macrophage responses. Interestingly, this corona-dependent inflammation was not observed, or greatly diminished, at later timepoints of disease despite high levels of circulating inflammatory mediators. Through this, we identified a panel of pro-inflammatory plasma biomolecules that become enriched on the nanoparticle surface at early stages of inflammatory disease progression.
These results highlight how pre-existing conditions are a critical factor that should be considered in nanoparticle-based drug development landscape. Hosts’ inflammatory status could inadvertently turn nanoparticle therapies into pro-inflammatory triggers, potentially leading to unintended consequences. While our study was limited to mice, future work will focus on validating these findings in various inflammatory human diseases with clinically relevant nanoparticle formulations. Ultimately, we aim to discover biomolecules that predict how nanoparticles will behave in different patients, helping to guide the development of future nanomedicines.
Written by:
Jacob R. Shaw, Ph.D. and Ryan M. Pearson, Ph.D.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in