Unveiling the Hidden Inner Architecture of Biomolecular Condensates
Published in Bioengineering & Biotechnology, Chemistry, and Materials
Cellular location is critical for the fate of the molecules of life. Often, RNA and protein molecules reversibly condense—just like water molecules when clouds form—into membraneless organelles that organize the plethora of chemical reactions the cell has to perform. For years, we pictured the inside of these biomolecular condensates—tiny warehouses and factories—as bustling, uniform environments where molecules move, i.e., diffuse, freely. Our new study now shows that these cellular compartments instead harbor a nanoscale architecture that dictates movement and retention. This discovery is reshaping our understanding of basic cellular biochemistry and the progression of neurodegenerative diseases.
The Challenge of Imaging at the Nanoscale
Observing the inner workings of biomolecular condensates is no simple feat. We faced three major hurdles in our study.
The Motion Problem: Condensates are in constant motion, making it difficult to distinguish the movement of molecules inside from the movement of the entire condensate.
Keeping it Real: In the cell, condensates are mostly spherical. But traditional in vitro reconstitution techniques often caused them to flatten against surfaces, distorting their shape and introducing unwanted protein-surface interactions.
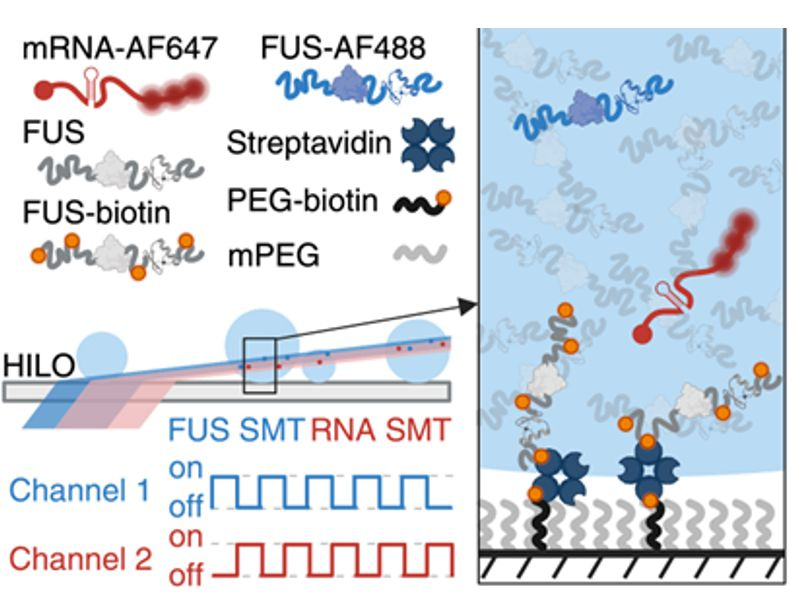
Zooming In: To understand how molecules organize within condensates, we needed to image internal structures at the nanometer scale while also tracking the rapid movement of individual molecules.
To overcome these obstacles, we developed a new approach. By tethering the condensates to a treated slide surface, we could hold them steady without altering their spherical shape. This stable platform allowed us to use high-resolution single-particle tracking to map the trajectories of thousands of individual protein and RNA molecules. We picked FUS—a nuclear RNA-binding protein involved in cancer and neurodegeneration—mixed with a vanilla mRNA as a model system.
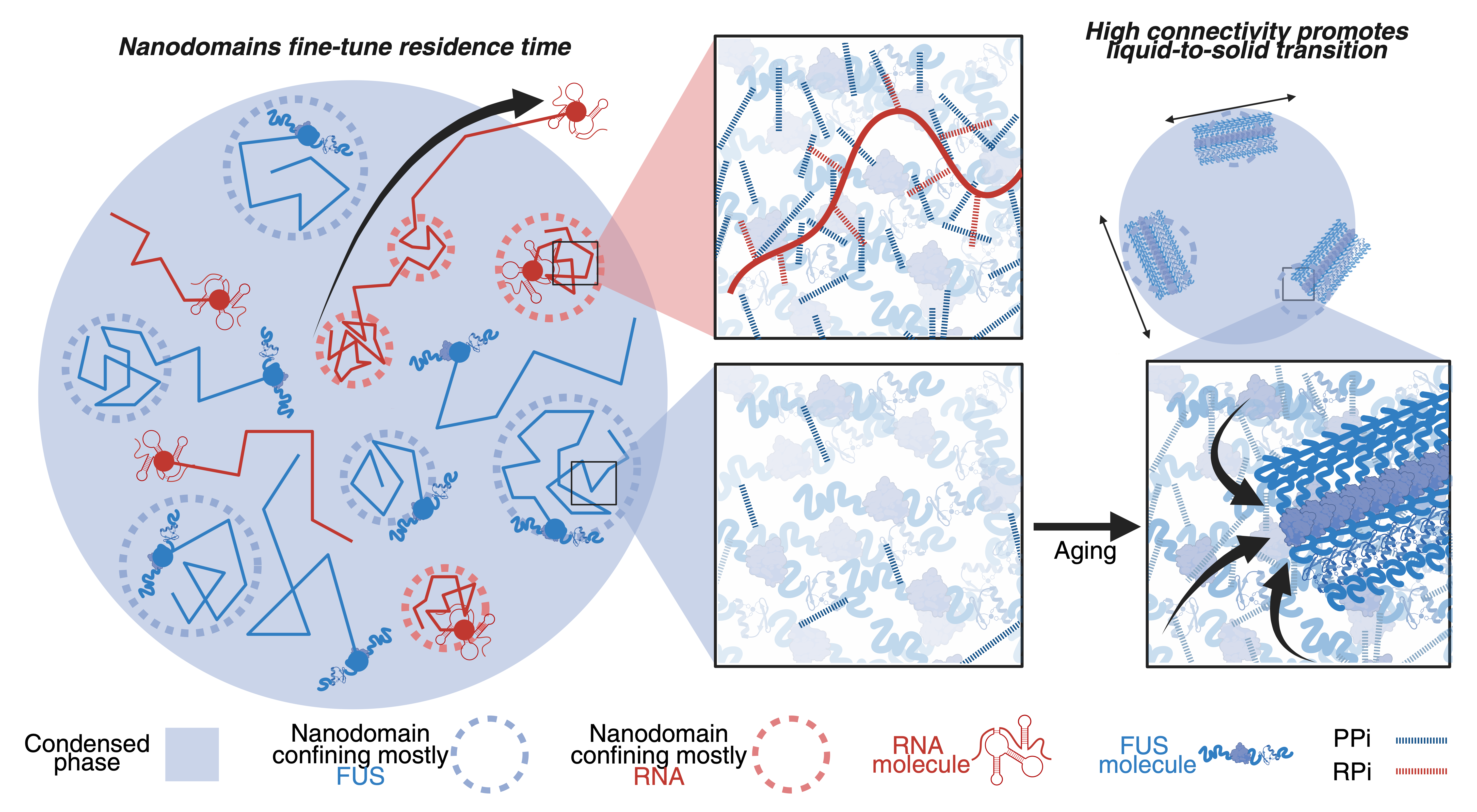
Discovering Nanodomains as Molecular Traps
What we found was unexpected. A large percentage of the FUS proteins (about 67%) and mRNA molecules (around 83%) were not moving freely, but were instead confined to distinct nanoscale regions we dubbed "nanodomains." These aren't separate droplets within the condensate, but rather localized zones within the condensate's network where movement is slowed.
These nanodomains, sized between 100-200 nm, are relatively immobile. FUS and RNA nanodomains create unique chemical environments and, interestingly, occupy separate, minimally-overlapping spaces.
By trapping molecules, nanodomains increase the time spent within the condensate, allowing condensates to retain and control diffusion of their composite molecules. This extended "residence time" may allow for prolonged storage or more efficient processing depending on the guest molecules present, suggesting they are adaptable, organizational structures that can be tuned for specific needs.
From Internal Organization to Liquid-to-Solid Transition
Our investigation took a fascinating turn when we explored the connection between this internal architecture and the "aging" of condensates—a process linked to neurodegenerative diseases. Over a 24-hour period, we observed a dramatic relocation of the nanodomains from the interior to the surface of the condensates.
This migration may be a crucial step in the formation of solid fibrils, a hallmark of neurodegenerative diseases like amyotrophic lateral sclerosis (ALS). Surface accumulation may create an environment that facilitates FUS protein in the nanodomain to misfold and aggregate into these harmful fibrils, linking the internal structure of the condensate to the liquid-to-solid transition observed in neurodegeneration.
A New Perspective on anti-ALS Drugs
This led us to a surprising observation. When we treated the condensates with two FDA-approved ALS drugs, Riluzole and Edaravone, we expected to see a slowdown of this nanodomain migration. Instead, both drugs actually increase the positioning of nanodomains to the surface, potentially promoting the formation of fibrils.
This result supports the hypothesis that these drugs perhaps facilitate protein aggregation, removing the most toxic small clusters of FUS proteins. By speeding up their conversion into solid, inert fibrils, these drugs could be sequestering the more dangerous forms of the protein, acting as a protective mechanism for the neuron. This shifts our focus from "inhibited aggregation" to "guided aggregation" as a potential therapeutic strategy.
What This Means for the Future
Our research reveals that the internal architecture of biomolecular condensates is a key regulator of their function, from everyday cellular processes to the progression into disease. This model of functional internal condensate architecture opens up exciting new avenues for research and therapeutic development. By understanding the principles of nanodomain formation and movement, we may one day be able to harness it for the design of synthetic condensates for drug delivery or for developing new treatments that specifically target the organization of these condensate sub-structures. The journey into the intricate nanoscale organization within our cells is far from over, but with each new discovery, we move closer to unraveling the complex mysteries of life and disease.
Follow the Topic
-
Nature Nanotechnology

An interdisciplinary journal that publishes papers of the highest quality and significance in all areas of nanoscience and nanotechnology.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in