WHEN TARGETED THERAPY MEETS IMMUNOTHERAPY. DASATINIB PLUS BLINATUMOMAB DRIVES A DISTINCT IMMUNE SIGNATURE IN FRONTLINE PH+ ACUTE LYMPHOBLASTIC LEUKEMIA (Ph+ ALL)
Published in Cancer, Biomedical Research, and Immunology
 Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL), driven by the BCR::ABL1 fusion has been historically characterized by an aggressive disease and a very unfavorable outcome. Prior to the use of tyrosine kinase inhibitors (TKIs) it was the hematologic malignancy with the worst prognosis. In the past 25 years with the advent first of TKIs and, more recently, the addition of immunotherapy we have witnessed a revolution in the management and outcome of Ph+ ALL patients of all ages.
Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL), driven by the BCR::ABL1 fusion has been historically characterized by an aggressive disease and a very unfavorable outcome. Prior to the use of tyrosine kinase inhibitors (TKIs) it was the hematologic malignancy with the worst prognosis. In the past 25 years with the advent first of TKIs and, more recently, the addition of immunotherapy we have witnessed a revolution in the management and outcome of Ph+ ALL patients of all ages.
One of the most practice-changing strategies has been the introduction of chemotherapy-free frontline approaches that combined a TKI with the CD19-directed bispecific T-cell engager blinatumomab. While both second and third generation TKIs (dasatinib and ponatinib) have a very marked anti-leukemic activity, we asked a clinically relevant question: do these regimens, in the absence of systemic chemotherapy, shape the host immune system in the same way when associated with blinatumomab—or do they generate a distinct immune status that may influence the depth and durability of response?
Our recent study, published in Leukemia, directly addresses this issue by comparing the immune modulation induced by dasatinib + blinatumomab compared to ponatinib + blinatumomab in newly diagnosed adult Ph+ ALL patients.
Why look at the immune modulation in the first place?
Blinatumomab is designed to redirect T cells against CD19+ leukemia cells, but its clinical activity depends on more than target engagement alone. The broader immune context—baseline immune competence, the balance between effector and regulatory compartments, and the status of innate immune populations—may have an impact on how well immune pressure is generated and maintained over time.
Dasatinib, in particular, has long been associated with immunologic effects (including lymphocytosis and expansion of cytotoxic subsets) in BCR::ABL1-driven diseases. We therefore hypothesized that the “TKI backbone” might not be immunologically neutral, especially when paired with blinatumomab.
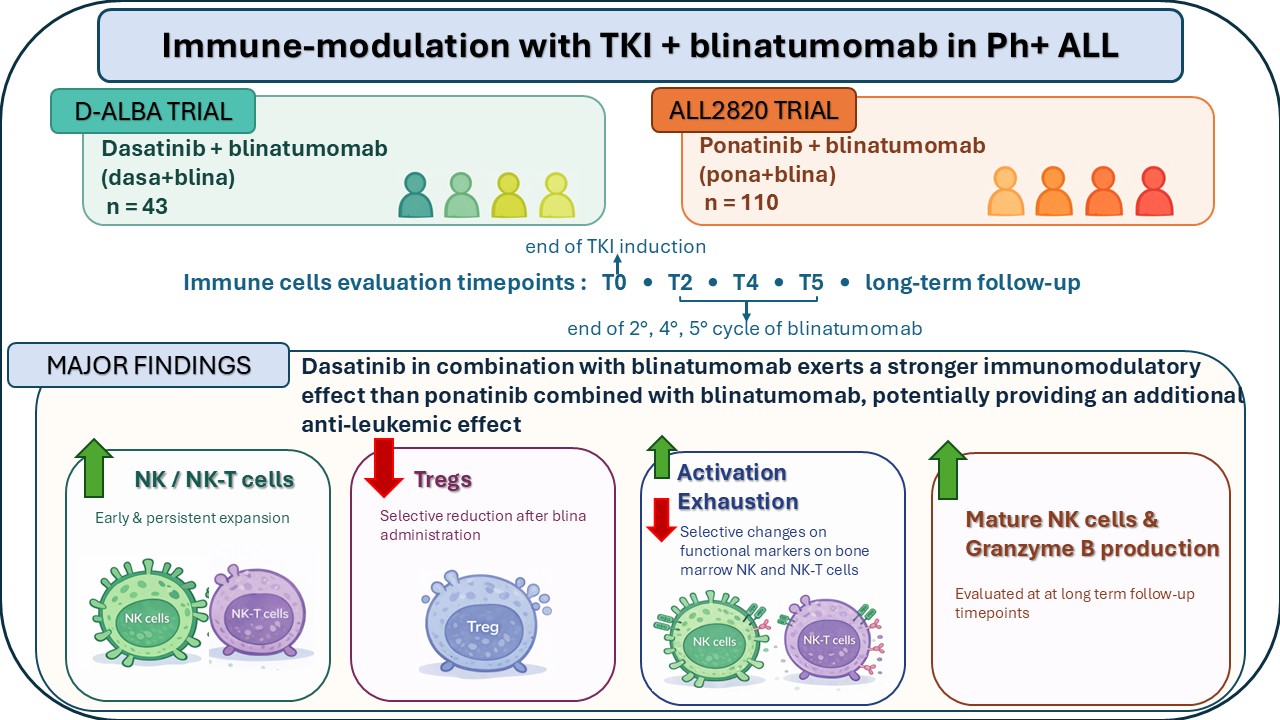
We analyzed serial immune monitoring data from 153 patients treated frontline in the GIMEMA LAL2116 (D-ALBA) and ALL2820 multicenter clinical trials:
- 43 receiving dasatinib + blinatumomab (dasa+blina)
- 110 receiving ponatinib + blinatumomab (pona+blina)
Immune cell subsets were evaluated in the peripheral blood at standardized timepoints:
- T0: end of induction
- T2, T4, T5: after 2, 4 and 5 blinatumomab cycles
In a subset, we also assessed bone marrow immune phenotypes (activation and exhaustion markers) and we complemented phenotyping with functional readouts of NK cell cytotoxic potential (perforin/granzyme B). Finally, we explored whether immune dynamics differed according to early molecular response (complete molecular response (CMR) at the end of induction).
Key findings
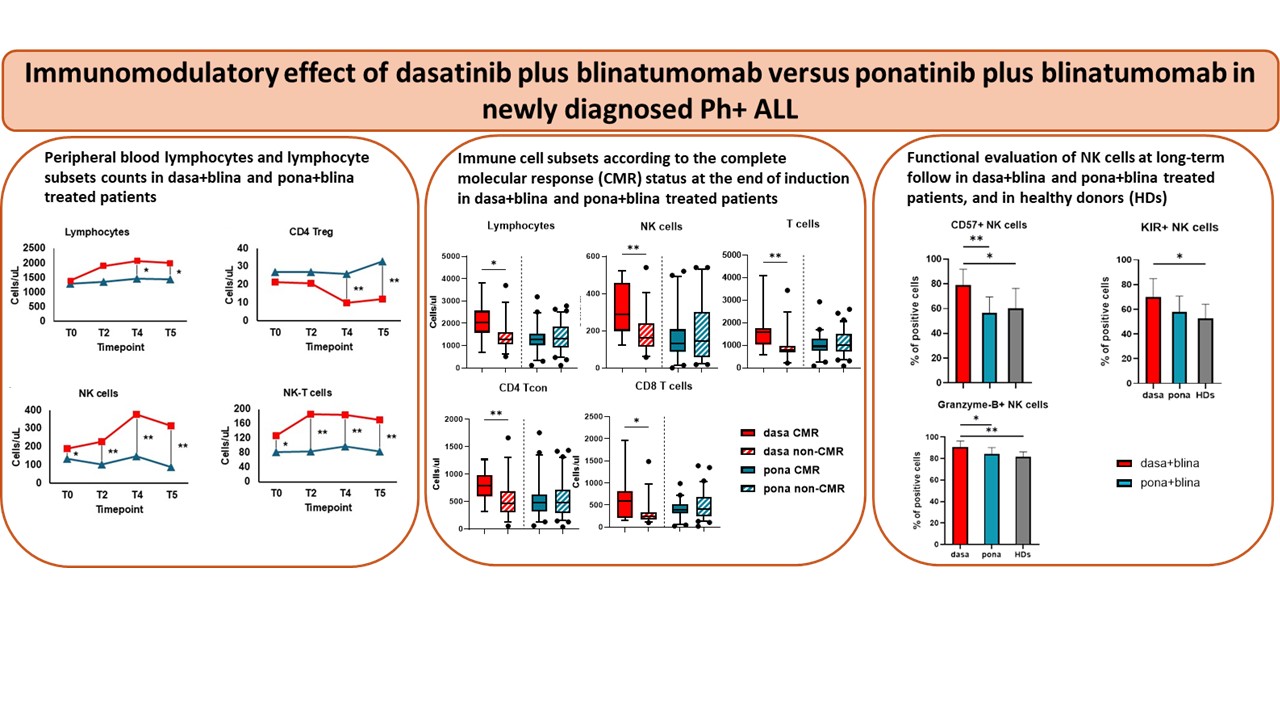
1) Dasatinib + blinatumomab induced an overall more potent lymphocyte expansion.
At later timepoints, the absolute lymphocyte count increased more in the dasa+blina cohort than in the pona+blina cohort, suggesting a regimen-specific synergy in sustaining lymphocyte expansion/survival over time.
2) Regulatory T cells (Tregs) declined selectively with dasa+blina.
A striking difference emerged in the regulatory compartment: Treg counts decreased only in the dasatinib-treated cohort, particularly after multiple blinatumomab cycles. Since Tregs can suppress anti-tumor immune responses, their reduction may facilitate a more permissive environment towards a sustained immune-mediated leukemia control.
3) NK and NK-T cells expanded early and persistently with dasatinib.
Innate immunity showed one of the clearest separations between regimens. NK and N-KT cell counts were higher in dasa+blina treated patients across all timepoints, and, importantly, differences were already visible at the end of induction—before full blinatumomab exposure—supporting an intrinsic immunomodulatory effect of dasatinib.
4) Bone marrow data suggest “more activated, less exhausted” NK/NK-T profiles with dasa+blina.
In bone marrow analyses, performed in a small group of patients, NK-T cells in the dasa+blina group showed higher activation markers (e.g., CD25, CD69) and lower exhaustion signatures (e.g., PD-1/TIM-3 co-expression) compared to the pona+blina cohort. Inhibitory/exhaustion markers (including KLRG1 and TIM-3/PD-1 patterns) were also comparatively reduced in NK cells of the dasatinib cohort, consistent with a more functional innate immune state.
5) Early molecular responders in the dasatinib treated patients showed an “immune advantage”.
Within the dasa+blina cohort, patients achieving a CMR at the end of induction had higher lymphocytes, T cells and NK cells at baseline compared to non-CMR patients—suggesting that an early molecular clearance may be associated with (or benefit from) a more favorable immune context that blinatumomab can further amplify. This relationship was less evident in the ponatinib cohort.
6) At a long-term follow-up, dasatinib was associated with mature NK phenotypes and ahigher granzyme B production.
In patients with a prolonged follow-up still receiving a TKI, those on dasatinib maintained higher NK frequencies, a more mature NK phenotype and a greater granzyme B production compared to ponatinib treated patients, supporting the concept of a durable innate immune imprint.
Why the immune modulation may be important?
Clinically, both TKI + blinatumomab strategies are highly effective, and the more potent anti-leukemic potency of ponatinib against BCR::ABL1 is well recognized. However, our findings indicate that the dasatinib + blinatumomab association generates a distinct immune signature characterized by:
- an expansion of the NK/NK-T compartments
- a selective reduction of Tregs
- activation/exhaustion patterns consistent with an improved immune fitness
- a durable innate immune functional capacity
This overall profile may represent an underappreciated contributor to a long-term disease control—particularly relevant as Ph+ ALL is moving towards an always greater chemotherapy minimization, a restricted transplant use for many/most patients and the realistic prospect of treatment discontinuation strategies in deeply and prolonged responding individuals.
From a translational perspective, these data also support the idea that immune monitoring could become a meaningful companion biomarker, not only to describe immune kinetics but potentially to help identify patients most likely to sustain a long-term remission.
Our work suggests that the choice of TKI may influence more than the direct BCR::ABL1 inhibition—it may indeed shape the immune landscape in which blinatumomab operates. We hope that these data will encourage additional mechanistic studies and support rational immune-informed strategies for the optimization of the frontline management of Ph+ ALL patients of all ages.
Follow the Topic
-
Leukemia

This journal publishes high quality, peer reviewed research that covers all aspects of the research and treatment of leukemia and allied diseases. Topics of interest include oncogenes, growth factors, stem cells, leukemia genomics, cell cycle, signal transduction and molecular targets for therapy.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcement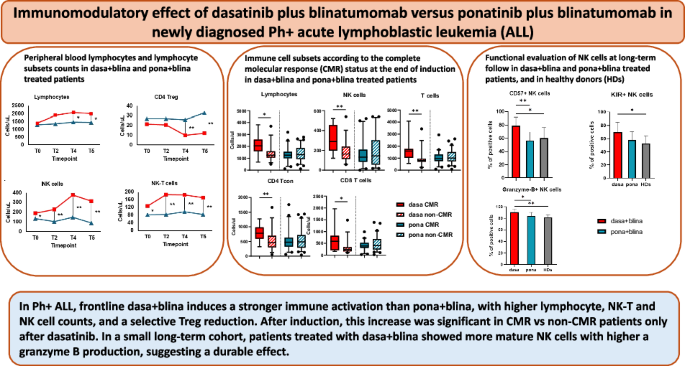





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in