Measure What (and When it) Matters: Building digital biomarkers for clinical trials
Published in Healthcare & Nursing

Drug development productivity for central nervous system (CNS) disorders has been steadily declining [1, 2], which impacts the rate at which new therapies become available to patients. One of the many factors that have contributed to this decline is the lack of sensitive, objective and high-resolution measures for assessment of disease progression. As a result, CNS clinical trials typically require larger sample sizes and have a higher risk of failure (well documented by Dorsey et al. [3]).
Digital biomarkers captured via mobile and connected technologies have the potential to transform clinical trials by moving assessments from the clinic to home, which can reduce patient burden while improving the quality of information (objective, more consistent, more frequent etc.) [4]. As we go from the clinic to home, two key challenges will need to be addressed:
-
Digital biomarkers must be clinically meaningful
- Assessments performed in the clinic are typically done in a highly controlled setting using very prescriptive tests. In contrast, those performed at home will involve a variety of situations and factors that can influence the measurement, increasing the variability. Not every measurement will provide clinically meaningful information. Therefore, a key aspect of capturing digital biomarkers at home will not only be what to measure but also when to measure. For example, gait measurements performed while the subject is carrying grocery bags will be very different from those measured when the subject is walking unconstrained.
-
Technology must be simple to use and scalable
- Clinical trials are complex endeavors with many different stakeholders (e.g. sponsors, regulators, CROs, clinical sites, patients and caregivers). For a new piece of technology to be successfully integrated into a clinical trial, it should be able to scale all the way from a single-site proof of concept study with 10s of subjects to a multi-site clinical trial with 100s or even 1000s of subjects. In addition, various stakeholders might have to learn to use a new piece of technology in a relatively short period of time, which will have a significant impact on the quality of information that will be gathered during a clinical trial. Therefore, developers will have to carefully consider the trade off between the system’s operational complexity (e.g. number of devices, steps involved in making a measurement) and capabilities (e.g. accuracy, types of measurements).
In this paper, we present an approach for processing raw accelerometer data from a single wrist-worn device to derive digital biomarkers of resting tremor and bradykinesia in patients with Parkinson’s disease (PD). One of the key drivers behind limiting the sensing modality to a single location (wrist on the most affected side) and sensor type (accelerometer) was to reduce complexity of the setup and operation of the device. The proposed system should be able to achieve continuous monitoring for days or weeks without needing patients to download data or recharge the device.
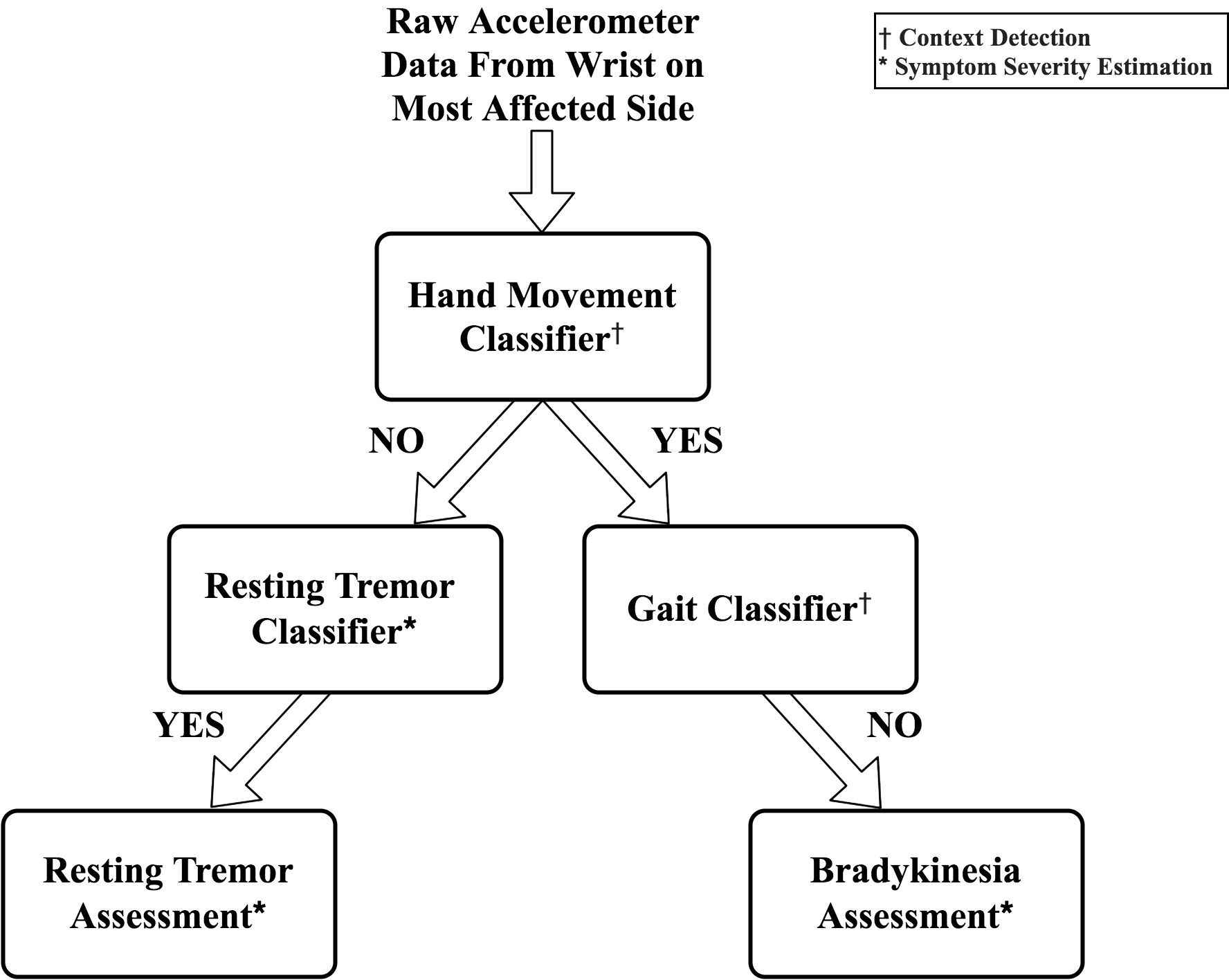
We took a pragmatic approach for algorithm development; using machine learning for more complex tasks (e.g. tremor and gait detection) and heuristic, rule-based algorithms for simpler tasks (e.g. hand movement detection). The system utilizes a hierarchical approach for sequentially processing raw sensor data by first performing context detection (hand movement and gait detection) followed by assessment of motor symptoms (resting tremor and bradykinesia). The motivation behind using this architecture was to provide more interpretable, context-specific measurements during ambulatory monitoring.
Results show that digital biomarkers derived during the performance of unscripted activities are clinically meaningful. Our conclusion is based on assessment of agreement with clinical ratings of symptom severity and sensitivity to detecting treatment-related changes in motor states (ON - well controlled motor symptoms and OFF - poorly controlled motor symptoms). Interestingly, agreement between clinical ratings provided by a live rater and those provided by video raters was poor; underlining the limitations of using remote video-based assessments as an alternative for assessments performed in the clinic.
Ultimately, a key value of using digital biomarkers is the ability to continuously monitor symptoms. However, this also comes with a challenge: how do we use continuous measurements for clinical decision making? We believe that a rethinking of the current paradigm, which is based on episodic assessments, is on the cards. To motivate this discussion, we provide an example that illustrates the granularity of information captured by continuous measurements.
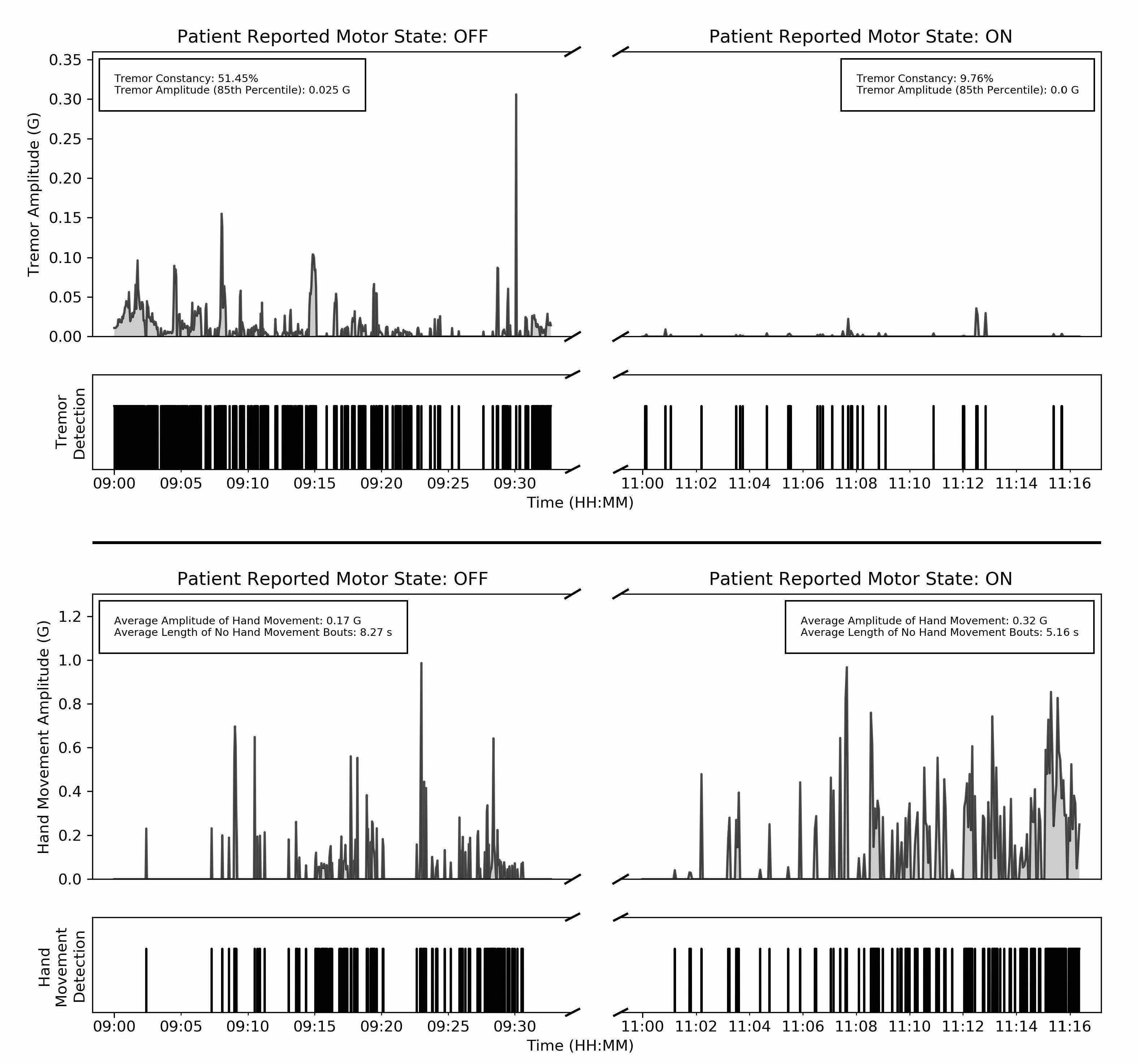
Looking Forward
The burden of CNS related disorders is expected to continue growing as life expectancy across the world keeps increasing. Digital biomarkers have the potential to not only accelerate the development of new therapies but also improve disease management resulting in better outcomes for patients. However, achieving broad adoption will require a concerted effort from various stakeholders, including patients, clinicians, engineers, designers and regulators, to develop solutions that are clinically valid and deployable at scale.
Authors:
Nikhil Mahadevan and Shyamal Patel
References
1. Scannell, J. W., Blanckley, A., Drug, H. B. N. R., 2012. (2012). Diagnosing the decline in pharmaceutical R&D efficiency. Nature.com, 11(3), 191–200. http://doi.org/10.1038/nrd3681
2. Pangalos, M. N., Schechter, L. E., & Hurko, O. (2007). Drug development for CNS disorders: strategies for balancing risk and reducing attrition. Nature Reviews Drug Discovery, 6(7), 521–532. http://doi.org/10.1038/nrd2094
3. Dorsey, E. R., Papapetropoulos, S., Xiong, M. & Kieburtz, K. The First Frontier: Digital Biomarkers for Neurodegenerative Disorders. Digit. Biomarkers 14642, 6–13 (2017).
4. Steinhubl, S.R., Wolff-Hughes, D.L., Nilsen, W. et al. Digital clinical trials: creating a vision for the future. npj Digit. Med. 2, 126 (2019) doi:10.1038/s41746-019-0203-0
Follow the Topic
-
npj Digital Medicine

An online open-access journal dedicated to publishing research in all aspects of digital medicine, including the clinical application and implementation of digital and mobile technologies, virtual healthcare, and novel applications of artificial intelligence and informatics.
Related Collections
With Collections, you can get published faster and increase your visibility.
Evaluating the Real-World Clinical Performance of AI
Publishing Model: Open Access
Deadline: Jun 03, 2026
Impact of Agentic AI on Care Delivery
Publishing Model: Open Access
Deadline: Jul 12, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in