A decision point between transdifferentiation and programmed cell death priming controls KRAS-dependent pancreatic cancer development
Published in Cancer and Cell & Molecular Biology
Pancreatic ductal adenocarcinoma (PDAC) has remained one of the most challenging cancers to treat, as even novel therapeutic approaches, such as immunotherapy or personalized treatment methods, have yielded disappointing results. We therefore wanted to delve deeper into this topic based on our expertise of programmed cell death mechanisms to identify a possible approach for the prevention and treatment of PDAC.
With the restrictions imposed by the coronavirus, the relocation of the lab to a new university and another move within the new campus shortly afterwards, the road to publication was far from easy. Despite these uncertainties and drawbacks, this work has continued to develop over time. Thanks to the excellent and scientifically high-quality collaborations, which were essential for the paper to become what it is today.
Acinar cells in the adult pancreas show high plasticity and can undergo transdifferentiation to a progenitor-like cell type with ductal characteristics. This process is known as acinar-ductal metaplasia (ADM). Persistent ADM is potentially harmful as it increases the ability of the transdifferentiated cells to develop into a PDAC. Using transgenic mice and primary cell and organoid cultures, we were able to show that transforming growth factor (TGF)-β-activated kinase 1 (TAK1), a kinase that regulates cell survival and inflammatory processes, prevents the elimination of transdifferentiated cells via two programmed cell death mechanisms, apoptosis and necroptosis, thereby enabling PDAC development. This suggests that a decision point between transdifferentiation and programmed cell death exists that controls PDAC development. Importantly, we were able to show that pharmacological inhibition of TAK1 induces PCD in patient-derived PDAC organoids. Therefore inducing rather than preventing PCD through TAK1 inhibition could hold a great translational potential and be a promising anti-cancer strategy for the prevention and treatment of PDAC.
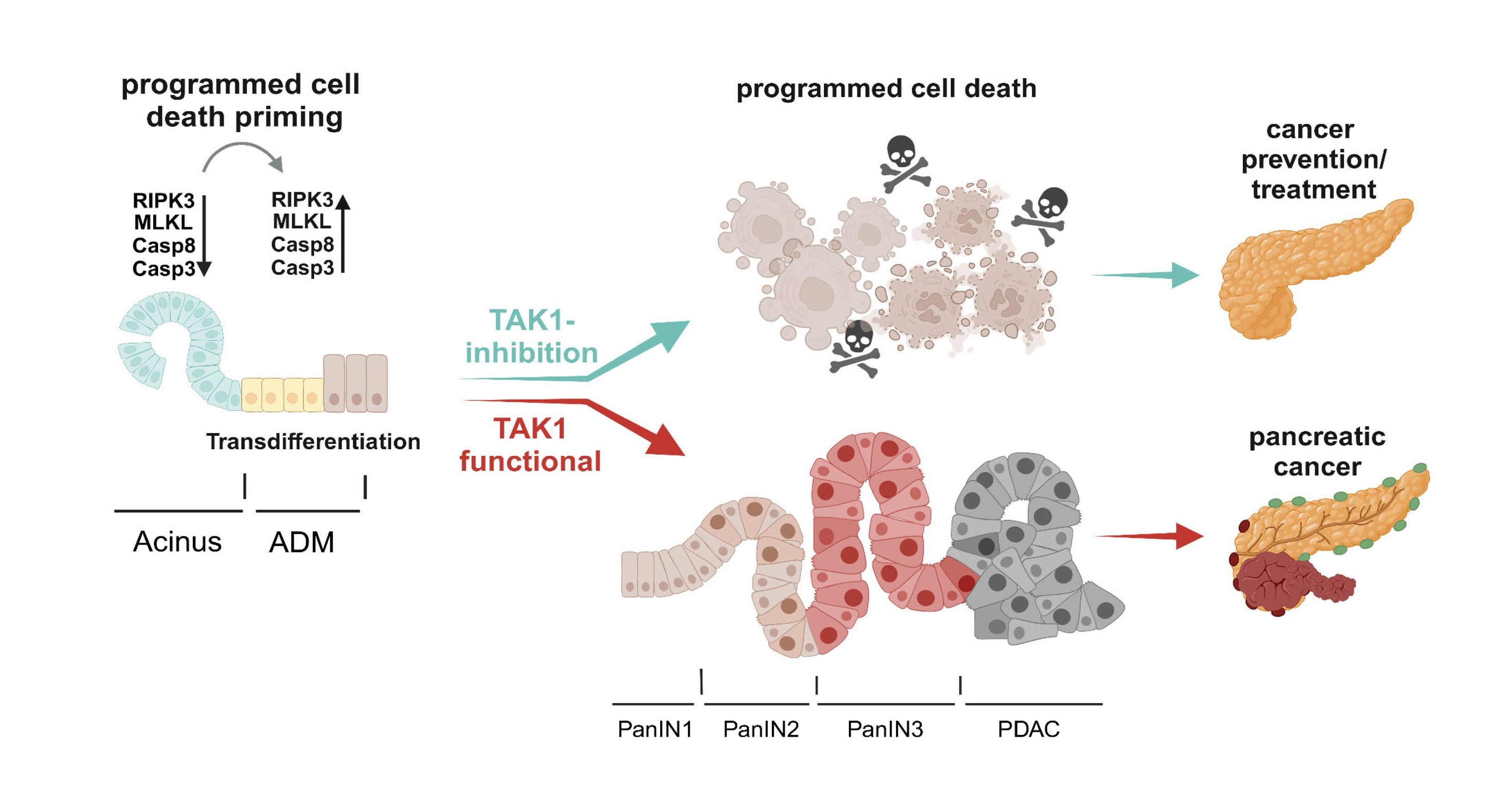
Figure 6. Proposed model on the role of TAK1 inhibition in KRAS-driven ADM and PDAC development. KRAS-dependent, and likely KRAS-independent, ADM induction leads to upregulated expression of PCD mediating molecules. Through its NF-κB-independent prosurvival functions, TAK1 prevents elimination of the PCD-primed transdifferentiated ductal cells, thereby enables PanIN formation and PDAC development. In contrast, TAK1 deficiency/inhibition impairs cell survival during ADM and prevents PanIN establishment and progression to PDAC. Created in BioRender. Schneider, A. (2025) https://BioRender.com/l87j101.
With this study, we were able to provide the basis for future clinical applications of TAK1 inhibition in the treatment of PDAC. We are continuing to work on this topic intensively and are excited to see how this research further develops.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in