A “Gourmet Diet” for Thermophilic Archaea: How Branched-Chain Amino Acid Specialization Drives the Diversification of Calditenuaceae
Published in Ecology & Evolution and Microbiology
In extreme environments, survival often depends on highly specialized metabolic strategies. One of the most iconic examples is the Great Boiling Spring (GBS) in Nevada, USA—a circumneutral geothermal ecosystem (~95.5 °C) that harbors abundant but largely uncultivated thermophilic archaea. For decades, the inability to cultivate many dominant archaeal lineages has limited our understanding of their physiology and ecological roles.
This long-standing challenge has now been addressed by an international research team led by Brian P. Hedlund (University of Nevada, Las Vegas). In a study published online in Nature Communications on February 4, 2026, the authors combined genome-guided cultivation with multi-omics and single-cell isotope tracing to uncover the metabolic logic and evolutionary history of the thermophilic archaeon Calditenuis ramacidaminiphagus (meaning “branched
-chain amino acid eater”).
From “uncultivable” to metabolically decoded
Genome-based surveys revealed that C. ramacidaminiphagus constitutes a substantial fraction of archaeal communities in GBS sediments (up to ~83.7% of the total archaeal population at 85 °C), indicating a high degree of ecological success. Yet paradoxically, this organism had remained refractory to laboratory cultivation.
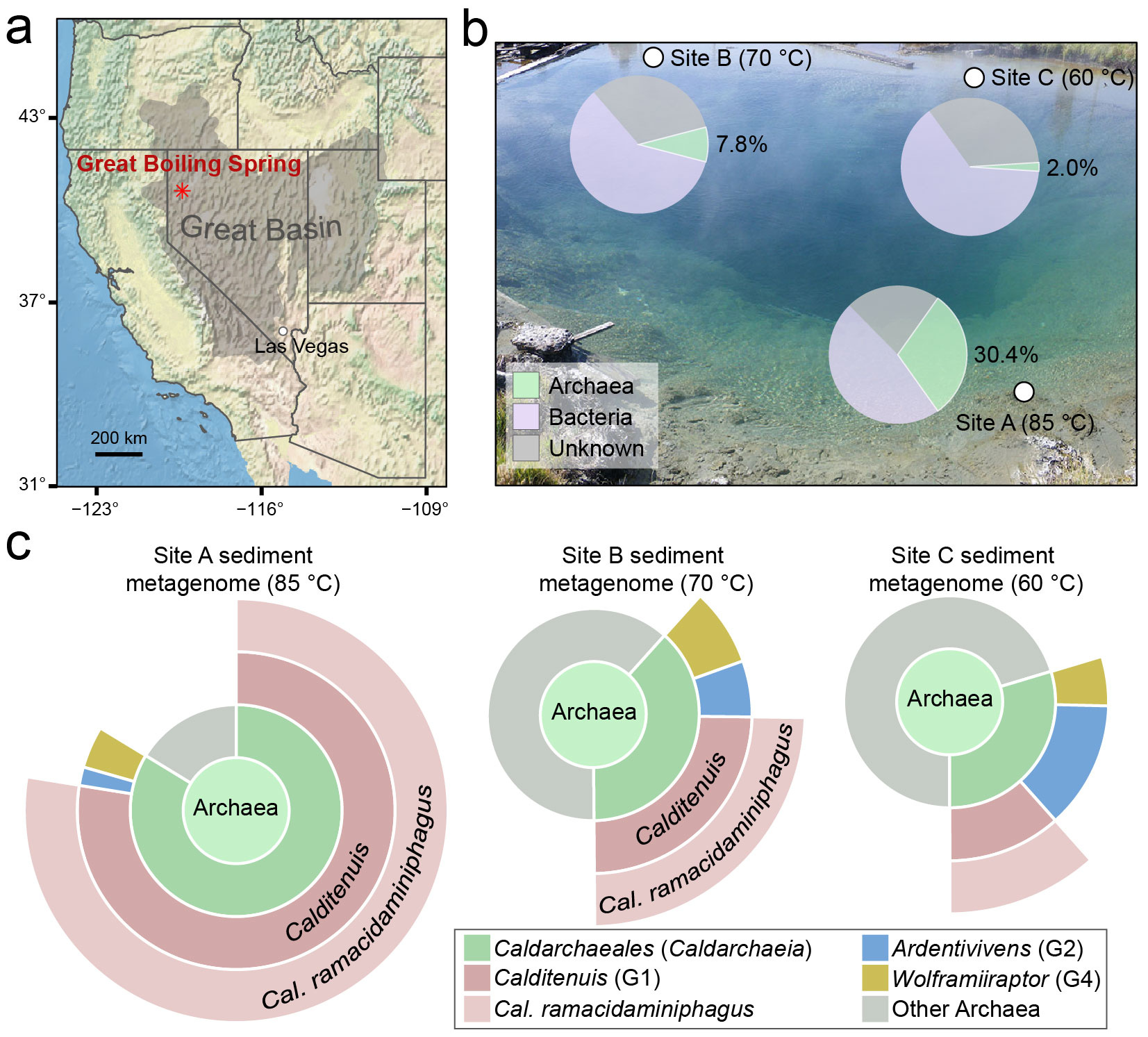
To directly probe its in situ metabolism, the researchers applied fluorescence in situ hybridization coupled with nanoscale secondary ion mass spectrometry (FISH–nanoSIMS). When incubated with ^13C-labeled branched-chain amino acids (BCAAs), cells of C. ramacidaminiphagus showed significantly higher ^13C enrichment than coexisting microorganisms, providing direct evidence that AAs are a preferred carbon and energy source in mixed communities.
Comparative genomic analyses revealed a striking metabolic bias. The genome of C. ramacidaminiphagus encodes an unusually large repertoire of BCAA transporters, while completely lacking transport systems for polar amino acids. This asymmetric genomic architecture points to an extreme degree of substrate specialization centered on BCAAs.
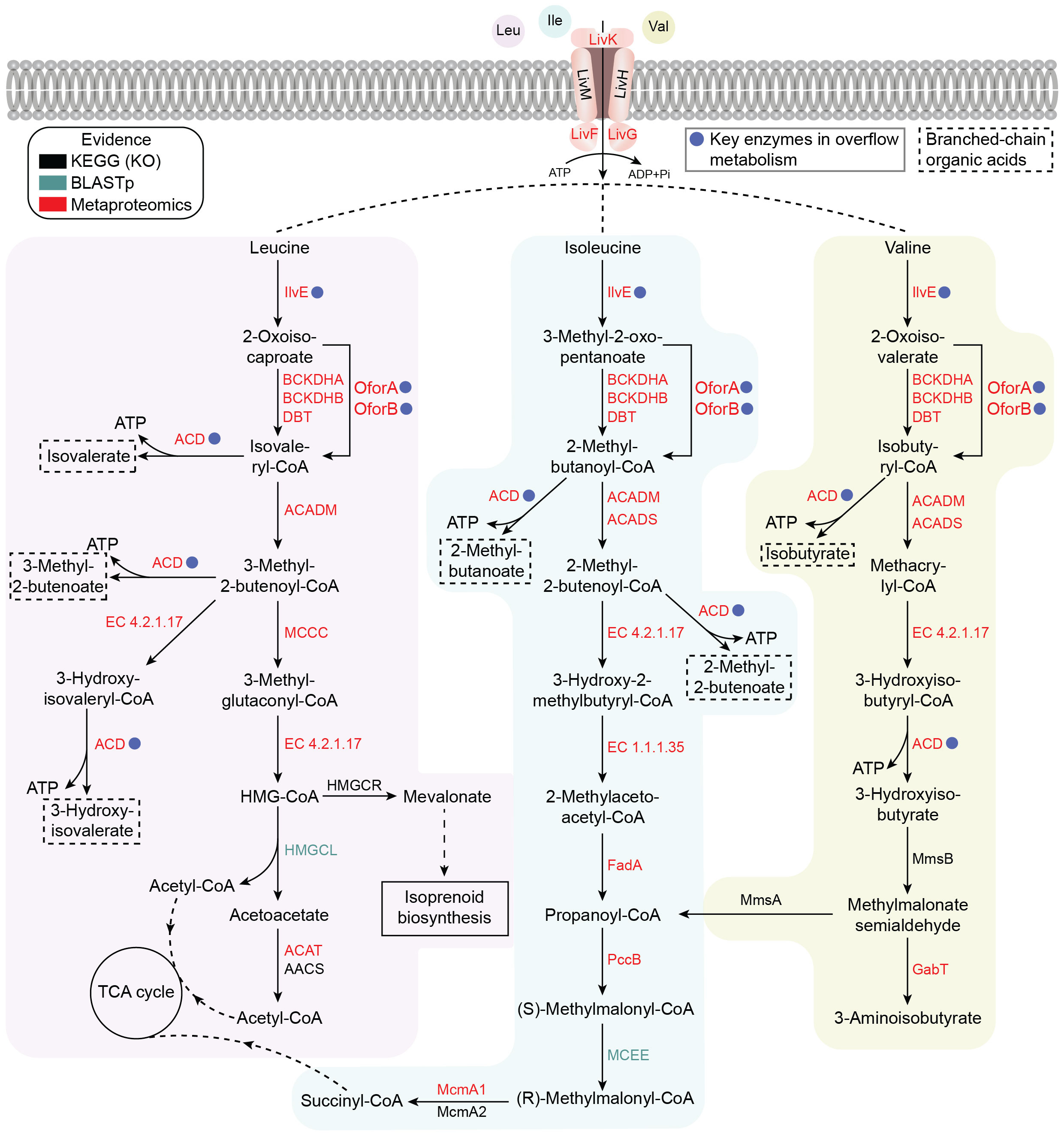
Metabolic reconstruction further showed that BCAAs play three central roles in this organism:
-
Structural function – serving as precursors for membrane lipid biosynthesis via short-chain fatty acid pathways;
-
Energetic function – being fully oxidized through the TCA cycle to support efficient ATP generation;
-
Overflow metabolism – enabling rapid energy acquisition through OFOR/ACD-mediated pathways under specific conditions.
Together, these functions define BCAAs as a multifunctional metabolic hub rather than a supplementary nutrient.
Genome-guided cultivation breakthrough
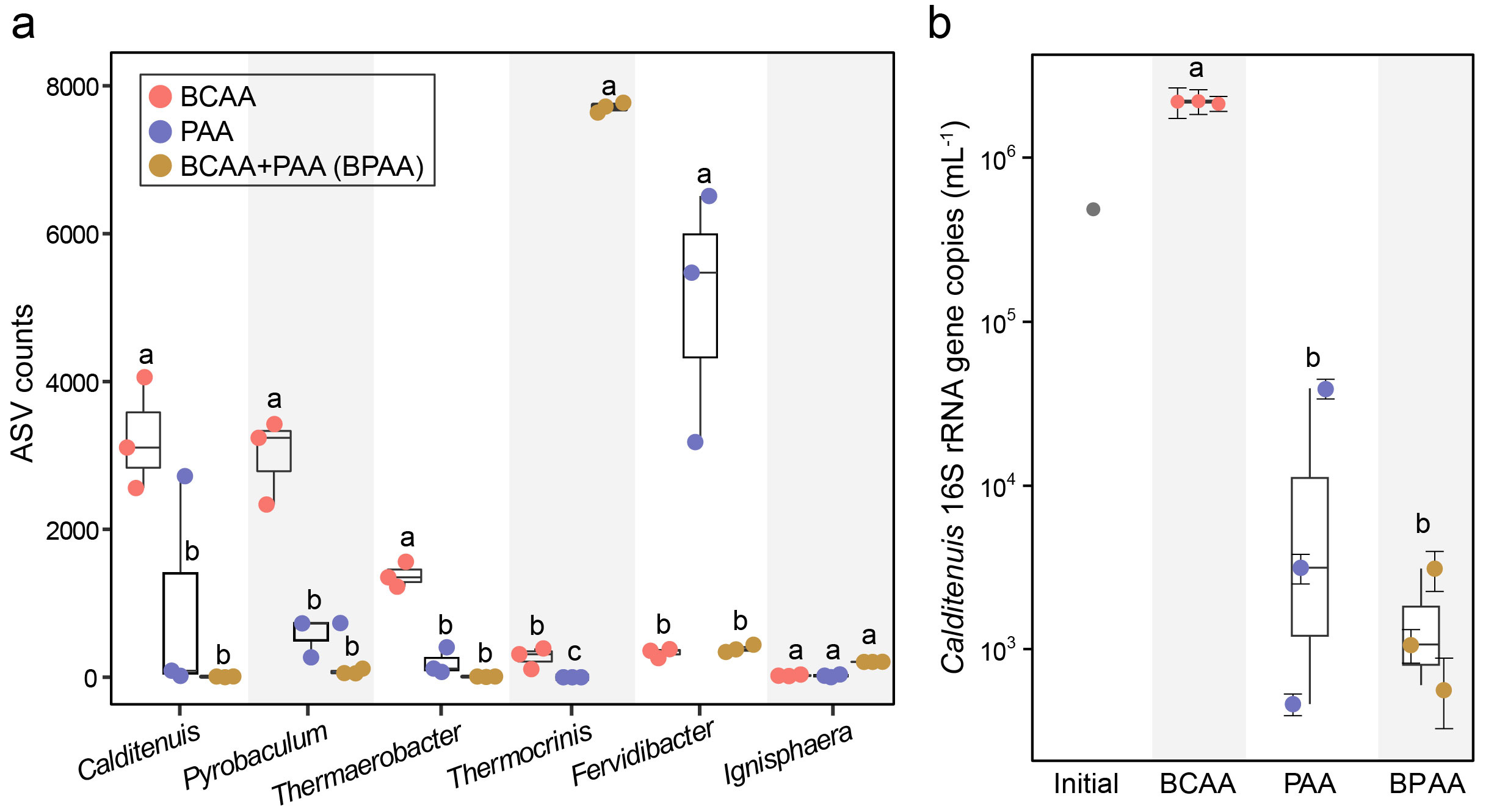
Guided by these insights, the researchers designed a defined “precision diet” medium in which BCAAs served as the sole carbon source. Under microaerobic conditions at 80 °C, C. ramacidaminiphagus rapidly proliferated, reaching an absolute abundance of 2.66 × 10^6 cells mL⁻¹ within seven days—a 278-fold increase compared with enrichment cultures. In contrast, replacing BCAAs with polar amino acids led to a sharp decline in abundance, experimentally confirming the organism’s strict metabolic specialization.
Evolutionary innovation via horizontal gene transfer
Beyond physiology, the study also reconstructed the evolutionary history of BCAA metabolism within Calditenuaceae. Phylogenomic analyses showed that key BCAA transporter genes were acquired through multiple independent horizontal gene transfer (HGT) events.
The ancestral lineage of Calditenuis appears to have assembled its BCAA utilization toolkit by repeatedly recruiting genes from the hot spring microbial gene pool. Subsequent gene duplication, rearrangement, and purifying selection expanded these transporters to 8–12 copies, forming a highly efficient and robust system finely tuned to BCAA-rich niches.
Ecological implications for hot spring ecosystems
This work paints a detailed picture of metabolic niche partitioning in geothermal environments, where dissolved organic carbon is scarce. Different microorganisms exploit distinct substrates: for example, Wolframiraptor gerlachensis preferentially utilizes sugars (Buessecker et al., NC, 2022), while Fervidibacter sacchari specializes in polysaccharide degradation (Nou et al., NC, 2024). In contrast, C. ramacidaminiphagus occupies a unique niche by scavenging BCAAs released from peptides and proteins degraded by other community members, such as Thermocrinis and Pyrobaculum.
Such specialization reduces direct competition and promotes stable coexistence, highlighting metabolism as a key axis of ecological organization in extreme environments.
Significance
By integrating genome-resolved ecology, single-cell isotope tracing, metaproteomics, and long-term cultivation, this study establishes a complete evidence chain linking gene acquisition, metabolic specialization, and ecological success. It demonstrates that horizontal gene transfer–driven metabolic innovation can fuel microbial adaptation and diversification in extreme ecosystems.
More broadly, the work challenges the traditional notion of “uncultivable” microorganisms and provides a blueprint for translating genomic predictions into cultivation strategies. The concept of a genome-encoded “dietary preference” underscores the central role of metabolism in microbial evolution, niche differentiation, and community assembly.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in