A new approach to malaria vaccination
Published in Microbiology
The paper in NPJ Vaccines is here: https://www.nature.com/articles/s41541-018-0068-2.pdf
Whole-sporozoite malaria vaccines
As the name suggests, whole-sporozoite malaria vaccines employ entire Plasmodium sporozoites, the form of the malaria parasite that resides in the mosquitoes’ salivary glands and is injected in a mammalian host during an infectious bite.
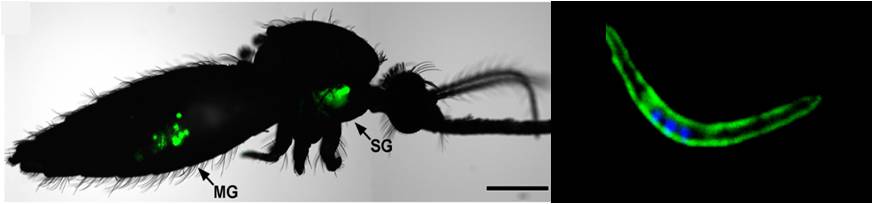
Plasmodium sporozoites are the mosquito salivary gland-resident forms of malaria parasites. Left: Plasmodium-infected mosquito. MG: midgut, SG: salivary glands, Image from Xu et al., PLoS ONE, 2013; Right: Plasmodium sporozoite. Image credit: António Mendes
In a natural infection, sporozoites infect and multiply inside liver cells, without any associated symptomatology. Pathology ensues once the newly formed hepatic parasites are released into the bloodstream and infect red blood cells, setting off malaria symptoms. Until now, whole-sporozoite malaria vaccination approaches employed human-infective P. falciparum sporozoites administered in an attenuated form. These parasites are fully capable of invading the liver cells, eliciting protective immune responses, while, provided attenuation is complete, they are incapable of infecting red blood cells and causing disease.
The roots of our idea
A few years ago, we had an idea for a different kind of vaccine against malaria. One that could potentially afford the known efficacy of whole-sporozoite vaccination, while ensuring complete safety without the need for parasite attenuation. Our idea draws from Sir Edward Jenner’s pioneering work, when he showed that inoculation of humans with the cowpox virus could afford protection against its related human pathogen, the smallpox virus.

Oil painting of Sir Edward Jenner. https://en.wikipedia.org/wiki/Edward_Jenner
Hence was born the first vaccine the world ever knew, and hence was planted the seed of our idea for a malaria vaccine: our proposal is to use a Plasmodium parasite that is not pathogenic to humans and tweak it to serve as a platform for vaccination against human malaria. The parasite is P. berghei, a rodent parasite species that does not cause malaria in humans, and the tweak is to genetically modify it to express antigens of its human-infective counterparts. The result is a non-pathogenic P. berghei parasite dressed in P. falciparum clothes that is expected to elicit a dual layer of immunity elicited by both cross-species and P. falciparum-directed immune responses.
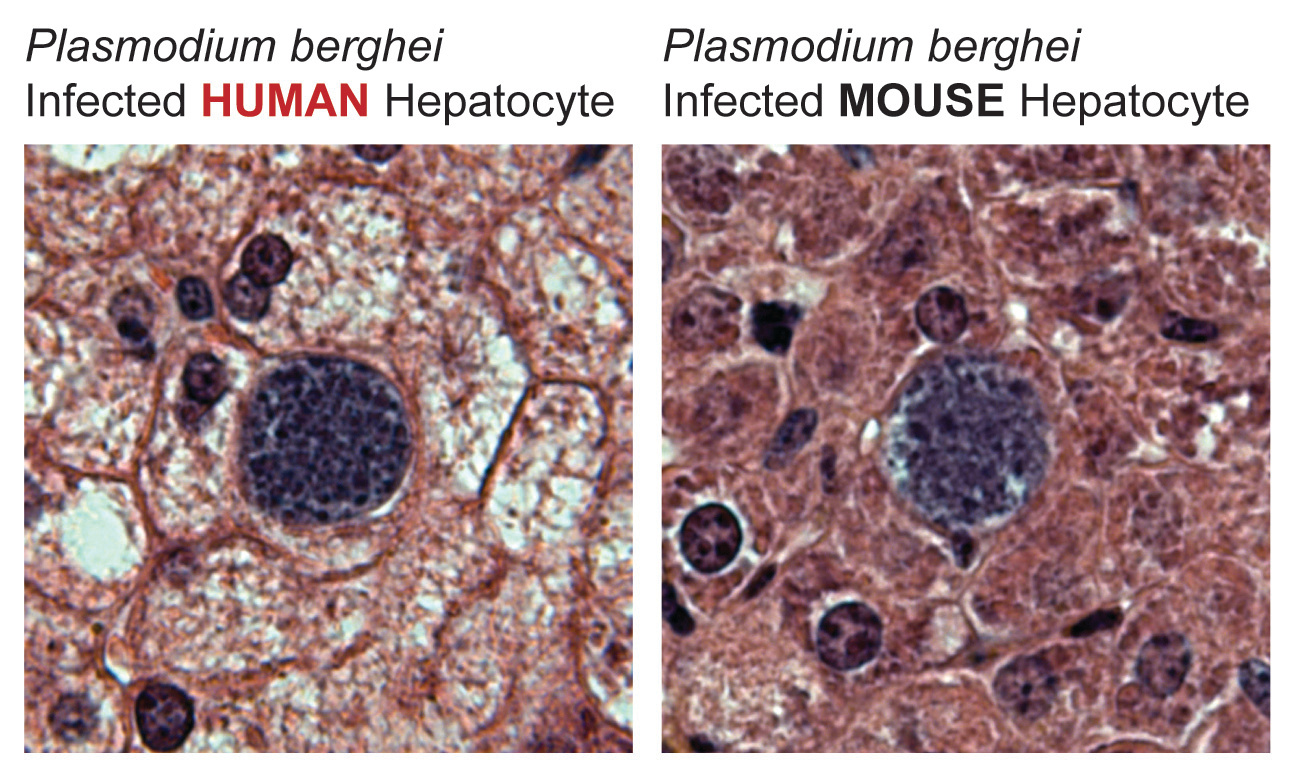
The rodent malaria parasite P. berghei is able to infect human hepatocytes in vivo, in the context of a liver-humanized mouse model.
The PbVac vaccine candidate: a P. berghei whole-sporozoite platform for antigen presentation
Enter PbVac, a genetically modified P. berghei parasite that expresses on its surface a major P. falciparum immunogen, the circumsporozoite protein. In this work, we show that PbVac meets all the requirements for a malaria vaccine. It infects human hepatocytes, as required for the generation of immunity and is incapable of developing in human erythrocytes, as required for safety. Crucially for our goals, we showed that it displays this exact same behavior in rabbits, allowing us to use this animal as an immunization model. Rabbit immunization experiments employing wild-type P. berghei and PbVac confirmed our initial hypothesis: PbVac elicits a combination of cross-species immune responses (mostly T-cell-dependent) and responses against the engineered P. falciparum circumsporozoite protein (predominantly antibody-based), the latter of which can functionally block liver infection by P. falciparum.
The PbVac malaria vaccine candidate: a genetically modified P. berghei parasite that expresses the P. falciparum circumsporozoite protein, a potent immunogen of the deadliest human-infective malaria parasite.
The future
Our work establishes an innovative concept of malaria vaccination, and places PbVac, the first member of this new family of malaria vaccine candidates, on the path to clinical evaluation.
Stay tuned…


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in