A novel nano-optogenetic approach for precision immunotherapy
Published in Bioengineering & Biotechnology

Chimeric antigen receptor (CAR) T-cell therapy has achieved exciting progress in both fundamental research and clinical applications over the last decade. CAR T-cell based therapy involves genetically modifying the patients’ T cells to express CAR molecules on the cell surface. A typical CAR contains a tumor-recognizing single-chain variable fragment (ScFv), and integrates essential components from both the T cell receptor (CD3ζ-ITAM) and co-stimulatory signaling (CD28 or 41BB) into a single polypeptide (the classical two-signal model; Figure 1). As a result, engineered CAR T cells can recognize the specific antigen on tumor cells to engage tumor cells in an MHC (major histocompatibility complex)-independent manner and be activated, allowing them to perform their tumor-killing functions. To date, at least five CAR T-cell therapy products have been approved by the Food and Drug Administration (FDA) in the US (Kymriah, Yescarta, Tecartus, Breyanzi, and Abecma) for clinical uses. However, according to the Phase II ZUMA-5 trial results 1, CAR T-cell therapy is often associated with side effects and faces significant safety challenges, as most notably exemplified by the cytokine release syndrome (CRS) due to uncontrolled production of pro-inflammatory cytokines and the “on-target, off-tumor” cytotoxicity. The latter side effect is related to the fact that CAR T cells attack not only tumor cells but also healthy cells or organs expressing the cognate antigen. There remains, therefore, a critical unmet clinical need for developing safer cell-based anti-cancer therapies.
We reason that the safety challenges associated with the 2nd generation CAR-T cell therapy might be partially tackled by precisely controlling the activity of engineered CAR T cells, allowing CAR T cells to be activated at specific time and locations on-demand.2 In this way, the “on-target, off-tumor” cytotoxicity and systematic CRS could be greatly mitigated by triggering CAR T cell activation only in the tumor sites. We set out to craft a wirelessly and spatiotemporally controlled CAR T toolkit. As a cross-disciplinary collaboration team (read more about Dr. Gang Han's group, Dr. Yubin Zhou's group, and Dr. Yun Huang's group), we have a unique combination of expertise in both nanophotonics, optogenetics and immune cell engineering. We have previously worked closely to develop nano-optogenetic approaches to control calcium signaling3-5 and immunogenic cell death6, such as pyroptosis and necroptosis, using wireless near-infrared light (NIR)-to-blue upconversion nanoparticles7. These successful examples encouraged us to further explore the idea of “nano-optogenetic immunotherapy”, in which photo-sensitive CAR T cells can be wirelessly controlled by NIR light.
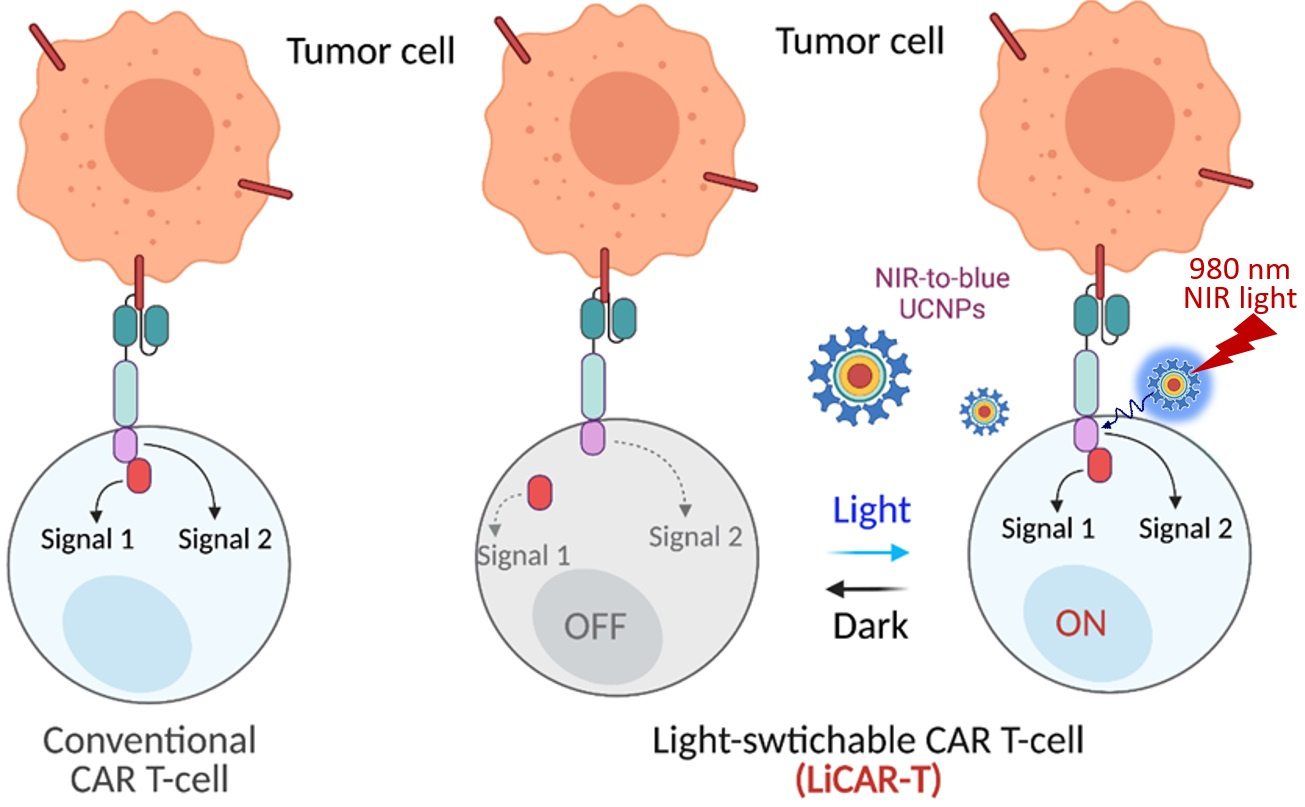
Figure 1. Nano-optogenetic immunotherapy using NIR light to wirelessly activate the LiCAR-T cells by NIR-to-blue upconversion luminescence.
In particular, we implemented the “nano-optogenetic immunotherapy” approach by genetically engineering the functional domains of CAR into two non-functional parts and installing photo-responsive modules into each part. The function of a split CAR can only be restored upon light-induced interaction between the two parts, thus constructing a light-switchable CAR (LiCAR; Figure 1). After going through multiple rounds of optimization to solve plasma membrane targeting, undesired nuclear accumulation, and background activation issues, we demonstrated that T cell expressing optimized LiCAR constructs showed antigen and light dually gated activation, thus attesting to the feasibility of our approach.
Moving one more step further toward in vivo applications, we have to address the tissue penetration problem facing blue light. Blue light is known to have a very limited depth of tissue penetration, with its intensity attenuated by over 95% when the thickness of human skin reaches 1 mm. One potential solution is to shift the photo-activation window toward the NIR range. NIR light is capable of penetrating deeper into the living tissues by up to a few centimeters, hence increasing the depth by over 1000-fold. We then come out with the idea of employing a special type of luminescent nanomaterial, namely upconversion nanoparticles (UCNPs). These tiny nanomaterials can effectively capture NIR light and emit bright blue light for local activation of LiCAR-T cells. Taking advantage of both the NIR-to-blue nanophotonics and genetically-engineered LiCAR-T cells, we could wirelessly control the activity of LiCAR-T cells with high spatial and temporal resolution.
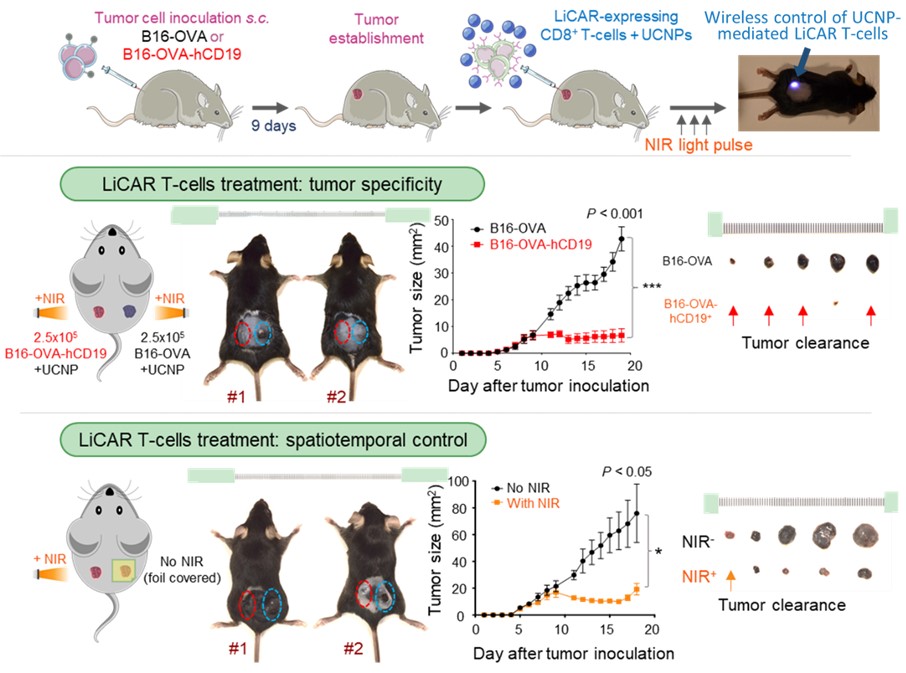
Figure 2. Schematic of wireless nano-optogenetic immunotherapy for cancer treatment in mice (top); LiCAR T cells showed high tumor specificity (middle) and enabled spatiotemporal control (bottom) for tumor clearance.
To validate our concept, we designed and expressed several receptors with genetically-encoded optical dimerizer pairs for the light-inducible assembly of spit CARs. Among them, we identified the best performing pair consisting of light-oxygen-voltage domain 2 (LOV2) and its binding partner sspB (the LOV2-ssrA/sspB pair) derived from oat. We next performed a series of cell experiments to validate their tumor-killing ability towards CD19-positive tumor cells. After confirming that our designed LiCAR-T cells showed high specificity to CD19 positive tumors in a strict light-dependent manner, we moved forward to the preclinical testing using both melanoma and lymphoma xenograft models. The UCNPs were either intratumorally injected or conjugated with LiCAR T cells for intravenous administration in the tumor-bearing mice. These UCNPs could serve as miniature nano-transducer or nano-illuminator to emit blue light in response to NIR irradiation, thereby allowing wireless control of LiCAR T cell activations in the tumor (Figure 2). Next, via a side-by-side comparison between conventional CAR-T and LiCAR-T cells in living animals, we concluded that the side effects associated with standard CAR T cell therapy, including CRS (reflected by IL-6 over-production) and B cell aplasia, could be effectively mitigated by using our nano-optogenetic LiCAR-T therapy.
It takes a long journey to screen, optimize and fine-tune the LiCAR-T cells, as well as test their coupling with UCNPs and in vivo performance with four different animal models. When we started the project in 2016, there was no FDA-approved CAR T-cell therapy. By the time this line of work was published, the approval number has grown to 5. We are devoting our efforts to craft better and safer CAR T-cell therapies, while also witnessing its successful growth. We believe that we are helping to shape the future. We anticipate that the nano-optogenetic immunotherapy represents a new direction of personalized cancer therapy, in which precise control of the timing, location, and dosage of T cell activation could be tailored to individual patients. More importantly, the attractive concept of nano-optogenetic immunotherapy should not be limited to the spatiotemporal activation of T cells. Given the recent success in engineering other immune cells and the modularity and high transferability of our approach, we can extend it to develop smart cell-based therapies based on natural killer cells, macrophages, or other cell types of therapeutic values.
The original article can be found in:
By Kai Huang & Nhung T. Nguyen
References
- Jacobson, C.A. et al. Outcomes in ZUMA-5 with axicabtagene ciloleucel in patients with relapsed/refractory indolent non-Hodgkin lymphoma who had the high-risk feature of early progression after first chemoimmunotherapy. Abstract #S213. Presented at the EHA2021 Virtual Congress, June 9-17, 2021.
- Nguyen, N.T. et al. Nano-optogenetic engineering of CAR T cells for precision immunotherapy with enhanced safety. Nature Nanotechnology (2021), DOI: 10.1038/s41565-021-00982-5.
- He, L. et al. Near-infrared photoactivatable control of Ca2+ signaling and optogenetic immunomodulation. eLife 4, e10024 (2015).
- He, L. et al. Circularly permuted LOV2 as a modular photoswitch for optogenetic engineering. Nature Chemical Biology 17, 915-923 (2021).
- Tan, P. et al. Optogenetic immunomodulation: shedding light on antitumor immunity. Trends in biotechnology35, 215-226 (2017).
- He, L. et al. Optogenetic Control of Non-Apoptotic Cell Death. Advanced Science 8, 2100424 (2021).
- Yu, N. et al. Near‐Infrared‐Light Activatable Nanoparticles for Deep‐Tissue‐Penetrating Wireless Optogenetics. Advanced healthcare materials 8, 1801132 (2019).
Follow the Topic
-
Nature Nanotechnology

An interdisciplinary journal that publishes papers of the highest quality and significance in all areas of nanoscience and nanotechnology.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in