A novel way to perform rapid diagnosis of bloodstream infections
Published in Microbiology
According to WHO, infections remain some of the most common causes of death [1]. In the US, at least 35,000 people die each year as a direct result of infections caused by antibiotic-resistant bacteria [2]. If no action is taken, by 2050, this number is projected to be 10 million people worldwide [3]. Frequent administration of broad-spectrum antibiotics within hospitals provides a strong selective pressure for the emergence of antibiotic resistance [4]. Of particular concern is the treatment of bloodstream infection (BSI). Although the bacterial concentration in BSIs is extremely low (c.a., 1-10 CFU/mL [5]), if not rapidly treated, BSIs may progress to sepsis and septic shock [6]. The current gold-standard diagnostics for BSI is based on bacterial culture. The process consists of three basic steps: (1) detection of bacteria by sample-culture, (2) identification (ID) of bacteria isolated from the culture and (3) antibiotic susceptibility testing (AST), which indicates the antibiotics that are most effective to kill the detected bacteria. The usual culture time for common infections is 16-48 hours, making the entire time-to-result as much as 74 hours or longer.
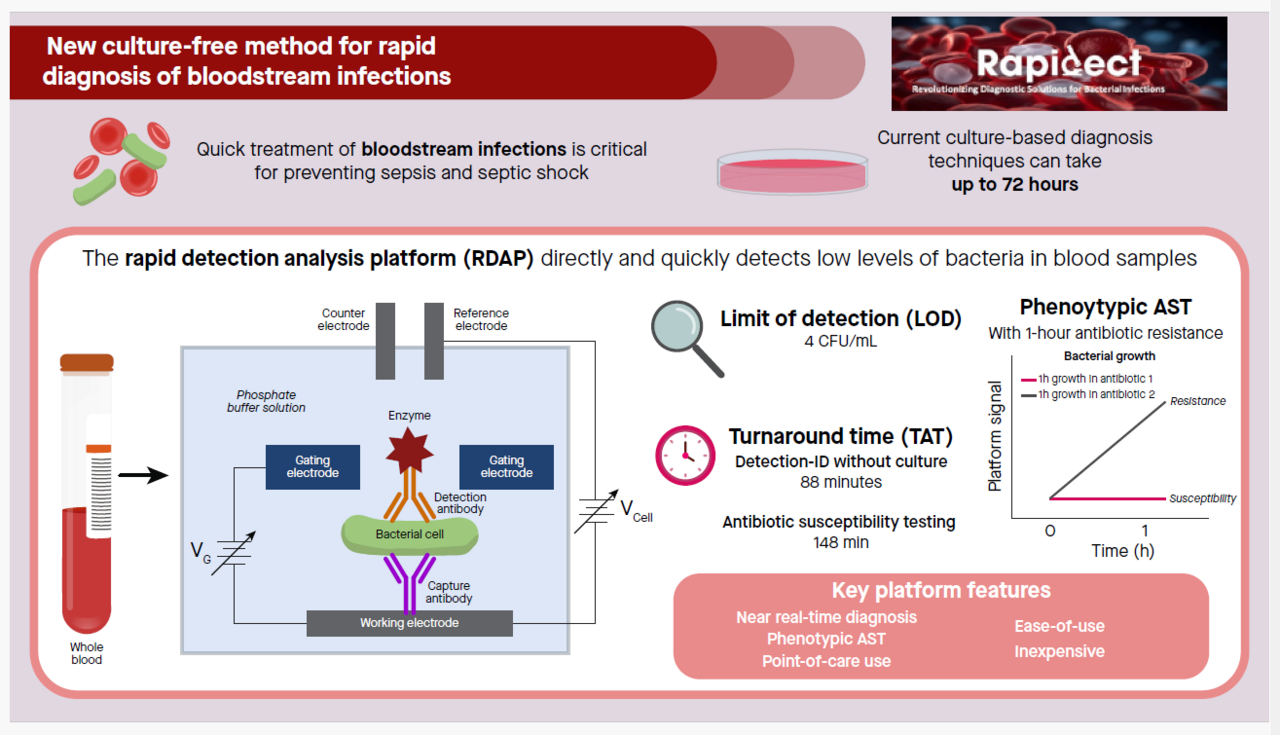
A challenging and unmet need in the treatment of BSI is providing targeted treatment using narrow-spectrum, bacteria-specific antibiotics that is desirably initiated in the very early stage of infections. Such treatment depends on the availability of rapid diagnostic technologies that provide near real-time diagnosis. We have developed the rapid detection-analysis platform (RDAP) [7,8], an electrochemical immunoassay platform with intrinsic signal amplification, for the diagnosis of BSIs. RDAP allows the direct detection of extremely low concentration bacteria in blood samples without culture and provides phenotypic AST. RDAP adopts disposable screen-printed electrodes (SPEs) as its detection electrodes. The current prototype features a limit of detection of 4 CFU/mL with a turnaround time (TAT) of 88 minutes for the combined detection-ID steps without culture and 148 minutes for AST with only 1-2 hours of antibiotic exposure.
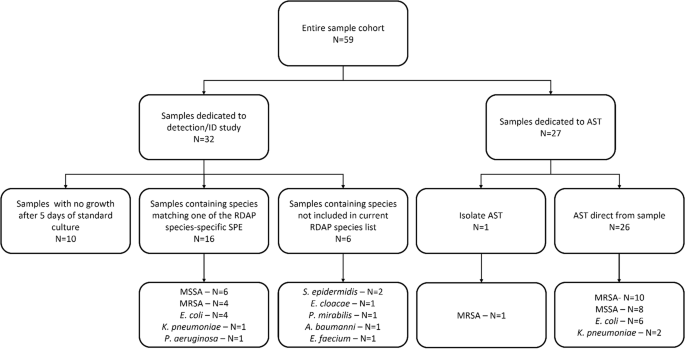
In our proof-of-concept study, we demonstrated, using contrived samples (E. coli spiked in blood), the feasibility of using RDAP to perform the three-step diagnostic workflow [7]. Recently, we have transitioned from contrived samples to real-world clinical samples and reported the diagnostic performance of RDAP on a cohort of 59 clinical blood samples [8]. The objective of this study was to perform our first clinical comparison of RDAP to current standard microbiology culture-based testing such as blood culture, matrix assisted laser desorption ionization time-of-flight (MALDI TOF) mass spectrometry and MicroScan. This study included detection and identification (N=32, 16 positive/16 negative) as well as AST (N=27). In this study, we showed the feasibility of using RDAP to rapidly diagnose BSIs.
In this study, we used RDAP to perform simultaneous detection-ID, which combines the first two steps of the diagnosis workflow into a single step, and AST. For each type of test, we presented the data for all the samples tested. The simultaneous detection-ID feature of the RDAP detects a particular bacteria in the sample and indicates the detection specificity against the other bacteria which are also the detection targets of RDAP. Seven bacteria-specific SPEs, namely, SPEs individually specific to E. coli, K. pneumoniae, P. aeruginosa, N. gonorrhoeae, methicillin-resistant S. aureus (MRSA), methicillin-sensitive S. aureus (MSSA) and S. pneumoniae were used to demonstrate simultaneous detection-ID. This range of bacteria represents 57% of all BSIs. Using a typical clinical microbiology laboratory diagnostic workflow that involved sample culture, agar plating, bacteria identification using MALDI TOF mass spectrometry as a clinical diagnostic reference, RDAP showed diagnostic accuracy of 93.3% for the detection-ID step. However, RDAP provided results at least 15 hours faster. Within the same time frame, RDAP also confirmed the negative culture results from the samples that had been tested negative (e.g. samples do not contain bacteria) using the laboratory methods.
The most salient feature of this work is that the RDAP demonstrated a novel AST approach for rapid diagnosis. The conventional method for AST is a two-step process. First, the bacteria in a sample are spatially isolated by culturing the sample with extremely low bacterial concentrations. Then, visual inspection is used to assign the identities to the separated bacteria and to transfer them into standardized solutions for the AST procedure, which is based on exposing the bacteria to different antibiotics. The AST provided by RDAP is a new type of antibiotic susceptibility metric based on antibiotics interaction pattern (AIP), which is a phenotypic approach to perform AST with a one-hour antibiotic exposure time. Using RDAP, we first performed AST measurements on a bacteria isolated from a clinical sample and spiked in a growth solution in order to demonstrate RDAP’s capability of producing AST results from isolated bacteria as required by the conventional method. We then took advantage of the ultrasensitive detection capability of RDAP to monitor the changes in bacterial concentration in response to antibiotics over very short time frames (i.e., 1 hour) to perform AST directly on the sample without bacteria isolation and standardization. The major part of our AST on clinical samples was performed using the direct AST approach with the goal of further reducing TAT. Using RDAP, we were able to achieve diagnostic accuracy of 95.4% for AST. However, RDAP provided results at least 15 hours faster than the standard microbiology testing adopted for routine lab diagnosis. The results of this study indicate an approach to provide near real-time diagnostic information for clinicians to significantly enhance the treatment outcome of BSIs.
Although the potential clinical utility of RDAP for culture-free diagnosis of BSI has been demonstrated, there are still several limitations and caveats of the platform in its current form. The results obtained so far were based on serial measurements. To fully exploit the culture-free approach, multiplexing capacity will be needed to make measurements on several samples simultaneously (i.e., parallel measurements). With multiplexing, the analytic time of the detection-ID and AST steps will remain at 88 minutes and 148 minutes, respectively. The TAT of RDAP represents a substantial potential improvement over standard commercial diagnostic technologies such as FilmArray, LightCycler and AcceleratePheno, which require positive culture of samples (confirmed presence of bacteria in the sample) as their input. So far, only seven bacteria (3 Gram-positive and 4 Gram-negative) have been considered. The number of bacteria-specific SPEs with antibody pairs will need to be expanded to include many more pathogens to achieve 95% of all BSIs. Finally, the AIP-based assignment of the sensitive-intermediate-resistant categories of the AST results produced by RDAP will need to be consistent with the MIC-based categories.
Based on the results from the clinical blood samples, we conclude that RDAP can (1) perform simultaneous detection-ID and AST on clinical samples containing common BSI-causing bacteria without culture enrichment; (2) achieve rapid and accurate phenotypic AST with 1-hour antibiotic exposure; and (3) perform AST directly from blood sample without prior species isolation. These features have the potential to dramatically reduce TAT and subsequently provide actionable results to clinicians, allowing the use of narrow-spectrum antibiotics before the second dose of empiric broad-spectrum antibiotics is given. Currently RDAP needs 88 minutes to perform the detection-ID step. Considering the 16 plus hours required by the standard lab procedure for this step, RDAP is already a near real-time diagnostic for the screening of certain specific species, e.g. MRSA, which is a test frequently ordered by clinicians. Further, antimicrobial de-escalation is a component of antimicrobial stewardship aimed to reduce exposure to broad-spectrum antibiotics. The fact that RDAP is able to detect negative culture samples in 88 minutes will limit the use of broad-spectrum to only the first dose and therefore effectively facilitate antimicrobial de-escalation in hospitals. As a final note, compared with polymerase chain reaction (i.e. PCR), a mainstream molecular diagnostic technology, RDAP is a potentially inexpensive detection-analysis technology with ease-of-use.
References
- Who, World Health Statistics 2020: Monitoring Health for the SDGs, Sustainable Development Goals, https://www.who.int/publications/i/item/9789240005105
- CDC, Antibiotic Resistance Threads in the United States, 2019 https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
- Walsh, F., Superbugs to kill more than cancer by 2050, https://www.bbc.com/news/health-30416844
- Backman, C., et al., An integrative review of infection prevention and control programs for multidrug-resistant organisms in acute care hospitals: A socio-ecological perspective, American Journal of Infection Control, vol.39, 368–378 (2011)
- Opota, O. et al., Blood culture-based diagnosis of bacteraemia: state of the art. Clinical Microbiology and Infection, vol. 21, 313-322 (2015).
- Seymour, C.W. et al, Time to Treatment and Mortality during Mandated Emergency Care for Sepsis, New England Journal of Medicine, vol.376, 2235-2244 (2017)
- Shi X, Kadiyala, U., VanEpps, J. S. & Yau, S.-T. Culture-free bacterial detection and identification from blood with rapid, phenotypic, antibiotic susceptibility testing. Scientific Reports 8, 3416 (2018).
- Shi X, Sharma S., Chmielewski R. A., Markovic M. J., VanEpps, J. S. & Yau, S.-T. Rapid diagnosis of bloodstream infections using a culture-free phenotypic platform. Communications 4, 77 (2024).
Follow the Topic
-
Communications Medicine

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary across all clinical, translational, and public health research fields.
Related Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Healthy Aging
Publishing Model: Open Access
Deadline: Jun 01, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in