A paper guide to how nuclear wrinkling unfolds
Published in Physics

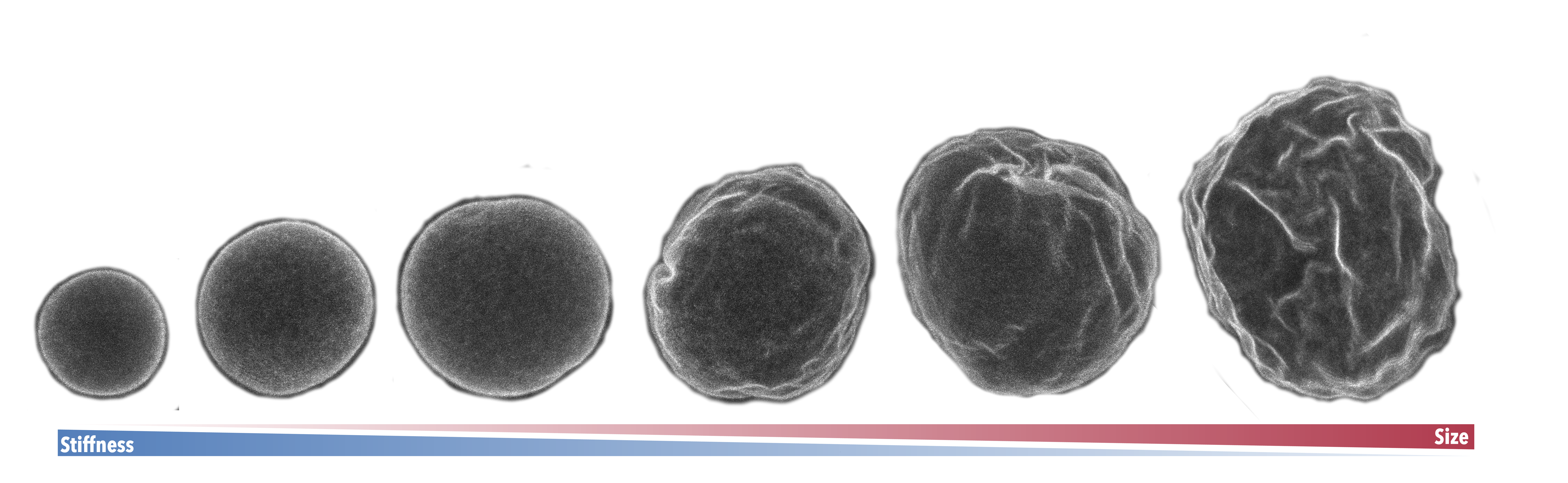
In 2021, Jonathan, Jasmin and I had been working together on understanding the role of mechanics in a process called ‘nurse cell dumping’ that unfolds during egg development in the fruit fly [1]. In flies, the egg does not form in isolation; rather, for most of its development, it is connected to ‘nurse cells’ that grow large by undergoing numerous cycles of DNA replication without cell division. As a result, the nurse cells’ nuclei, now with up to a thousand copies of the genome, become particularly large (up to ~50 micrometres). Towards the end of egg development, the nurse cells transfer most of their cytoplasmic contents in a dramatic transport event called ‘nurse cell dumping’ to the future egg cell, thus providing the egg with provisions for the future embryo. Jonathan and Jasmin noticed that prior to this transport process, nurse cell nuclei became progressively more wrinkled as they grew in size (Fig. 1). As we were wrapping up the ‘nurse cell dumping’ project, we knew we had found our next project. Wrinkles are ubiquitous: from skin to shirts, origami to tinfoil, they are a common albeit mathematically complex feature of the everyday physics of thin objects.
Here was another instance, of substantial biological relevance, occurring in an experimentally accessible system. The nucleus is the structure that houses the cell’s DNA, and it is bounded by a complex membrane known as the nuclear envelope. Although the nuclear envelope is often schematized as a passive container, it is in fact a complex object that is affected by the cell’s state, and can in turn affect how DNA is read out in response to internal and external forces. There is compelling evidence suggesting that the shape of the nucleus affects cell function [2, 3]; however, several challenges have limited quantitative studies of nuclear shape, its evolution during development, and its response to perturbations. It was our impression that, given our experience with this experimental system, we could advance understanding in this area by studying nuclear shape and dynamics in the nurse cells. Nurse cells are large, can be fluorescently labelled, can be kept alive for a few hours for imaging, and the egg chamber is amenable to perturbations - both genetic and mechanical.
Through confocal microscopy, Jonathan was able to capture the three-dimensional shape of hundreds of nuclear envelopes at various stages of the egg chamber’s development. These highly resolved images clearly demonstrated that while young nuclei looked smooth and spherical, older nuclei looked wrinkled and crumpled like raisins (Fig. 1). Live imaging also revealed that the wrinkles were dynamic: appearing and disappearing within minutes. With these promising preliminary data, we set out to answer the following questions: What led to this visual difference? What explained the wrinkles’ genesis and evolution? Was this a largely physical phenomenon, or is it the product of complex biochemical pathways?
To make any statement about the mechanics of the wrinkles, the first problem was to quantify the observed wrinkling patterns. If we see two different pieces of crumpled tin foil, how can we compare them, or tell them apart? For this, we turned to a very generic mathematical tool, namely spectral analysis. In its most common form, spectral analysis consists of analysing the Fourier transform of a signal to understand its properties. By using generalisations of the Fourier transform to the sphere, one can thus study the patterns and anisotropies living on the surface of a sphere in instances as diverse as meteorological formations [4], the cosmic microwave background [5] - or in our case, the deformation of the spherical nuclei.
By projecting the surface of the nuclei onto the spherical harmonic basis, we were able to obtain power spectra revealing the statistical properties of the wrinkles. As our membranes are excited by fluctuations, the resulting deformations have a characteristic power spectrum that contains information about the fluctuations and the nature of the membrane material. In fact, our measurements showed that wrinkles were always present independently of the age of the egg chamber, differing only by the size of their wrinkles as their development progressed – but not their pattern.
Our results were initially surprising: typically, biological membranes are described as liquid, with no elasticity to them. However, the power spectra we expect of liquid membranes were in disagreement with what we observed consistently across our experiments. Moreover, when deformed liquid membranes form blob-like protrusions [6]; instead, our wrinkles form ridges and valleys. It became increasingly evident that the nurse cells’ crumpled nuclei looked perhaps more like crumpled paper.
A sheet of paper, like a sheet of graphene or a mylar space blanket, is much thinner than it is wide. This seemingly obvious fact actually makes crumpled paper an unexpectedly complicated material. Due to this elongated geometry, stresses in such thin-sheet materials can easily resist stretching but have a much harder time countering bending deformations. This behaviour leads to the material having a strong tendency to resist changes in gaussian curvature, and paper thus tends to deform around a series of ridges that connect multiple nearly flat regions (Fig. 2).
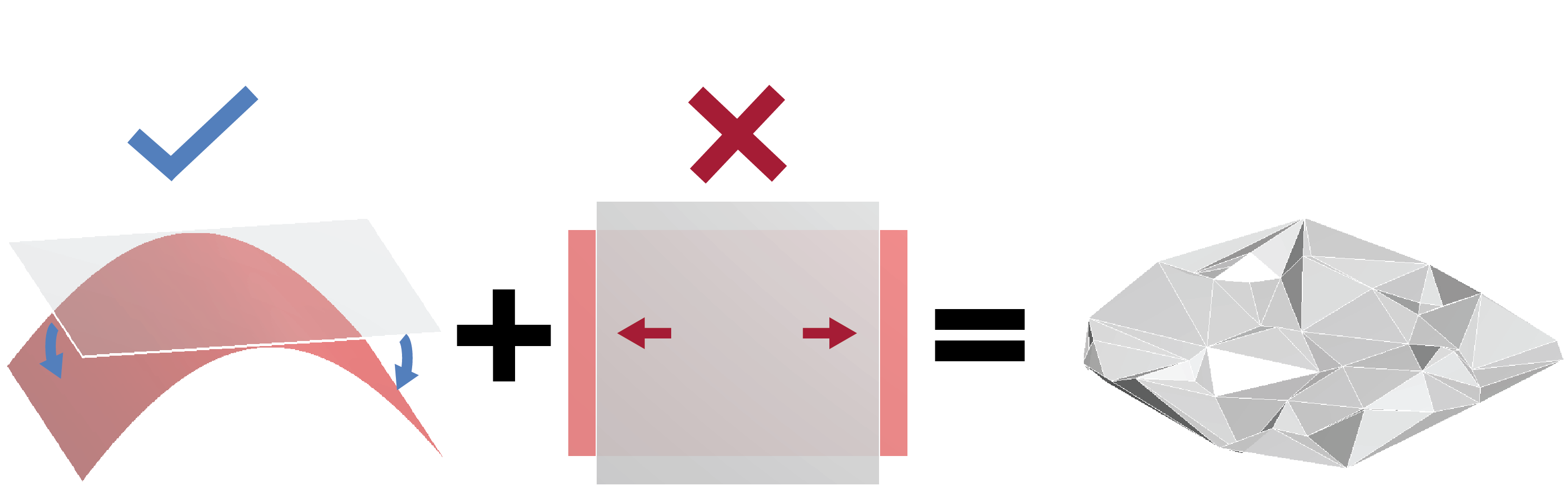
This description could just as well have applied to the wrinkles in the nuclear envelope, which is also very thin (its thickness is about one percent of the nuclear radius). Indeed, through two different theoretical approaches, we obtained a prediction for the power spectrum of a fluctuating paper-like sheet, which agreed well with our experiments, suggesting that thin-sheet elasticity is a reasonable model of the nuclear envelope [7].
We also found that the theory predicts that the pattern of wrinkles does not depend much on the details of the forces, as it is mostly set by the nonlinearity of the system. To test this prediction, we used osmotic shocks and drugs that inhibit some of the internal forces of the cell to change both the pressure levels and force patterns inside the cell, changing the amplitude but also likely the statistical distribution of stresses applied to the nuclear envelope. We indeed found, as theory predicted, that the size of the wrinkles changed, but not their pattern. This is remarkable: no matter how the nuclear envelope is driven, the deformations observed are similar. While we can only speculate, perhaps this phenomenon explains the robustness of mechanical sensing and other functions that are mediated by the shape of the nucleus.
To close, our work sheds light on the physics underlying nuclear shape and how physical laws, alongside biological processes, facilitate statistical regularity in the face of the complex and dynamic environment inside the cell. As physicists, we will want to better understand the nonlinear physics behind this statistical regularity, and as biologists we are curious how pervasive thin-sheet elasticity is in biological systems, how it affects function, and how increasingly answerable these questions are with ever-improving experimental and imaging techniques.
References:
[1] Imran Alsous et al, Proc. Nat. Acad. Sci. U.S.A. (2021)
[2] Almonacid et al, Dev. Cell (2019)
[3] Venturini et al, Science (2020)
[4] Bennett et al, ApJS (2013)
[5] Wieczorek and Meschede, Geochem. Geophys. (2018)
[6] Milner and Safran, Phys. Rev. A (1989)
[7] Košmrlj and Nelson, Phys. Rev. X (2017)
Follow the Topic
-
Nature Physics

This journal publishes papers of the highest quality and significance in all areas of physics, pure and applied.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in