A Promising Strategy for Solvent‑Regulated Selective Hydrogenation of 5‑Hydroxymethylfurfural over Porous Carbon‑Supported Ni‑ZnO Nanoparticles
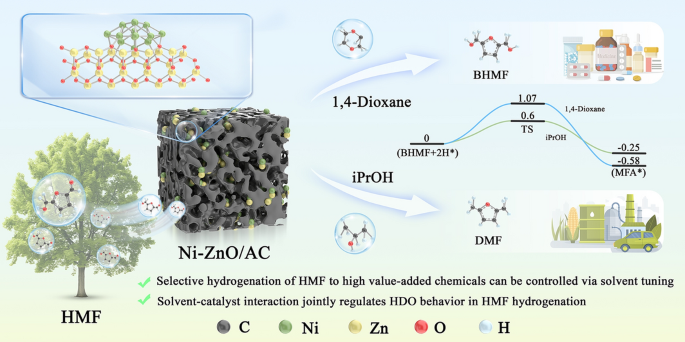
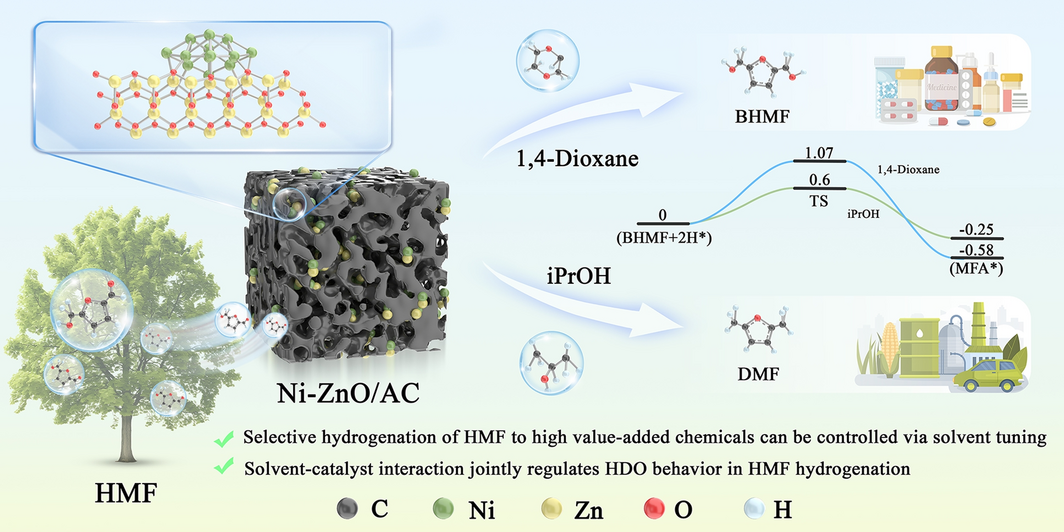
As the demand for renewable chemicals grows, selective conversion of biomass-derived platform molecules has become a key challenge. 5-Hydroxymethylfurfural (HMF), a promising biomass-derived intermediate, can be hydrogenated into valuable products such as 2,5-bis(hydroxymethyl)furan (BHMF) and 2,5-dimethylfuran (DMF). However, achieving high selectivity toward specific products remains difficult. Now, researchers from the Chinese Academy of Forestry and China University of Petroleum, led by Prof. Jianchun Jiang and Prof. Kui Wang, have developed a porous carbon-supported Ni-ZnO nanoparticle catalyst (Ni-ZnO/AC) that enables solvent-regulated selective hydrogenation of HMF with exceptional efficiency.
Why This Matters
- Tunable Product Selectivity: By simply switching solvents, the catalyst can selectively produce BHMF (97.5% selectivity in 1,4-dioxane) or DMF (99.5% selectivity in isopropanol).
- Green and Scalable: The catalyst is synthesized via low-temperature coprecipitation using coconut shell-derived activated carbon, offering a sustainable and cost-effective approach.
- Mechanistic Insight: The study reveals that solvent polarity and proton-donating ability jointly regulate the reaction pathway, with protic solvents enabling a hydrogen shuttle mechanism that facilitates hydrodeoxygenation.
Innovative Design and Features
- Ni-ZnO/AC Catalyst: A semi-core–shell structure with strong Ni-ZnO interfacial interaction enhances charge transfer and catalytic activity.
- Solvent Effect: Solvent polarity correlates linearly with product selectivity. Protic solvents like isopropanol lower activation energy (0.60 eV vs. 1.07 eV in 1,4-dioxane) by promoting proton-coupled hydroxyl removal.
- Stability: The catalyst maintains high activity and selectivity over five cycles with minimal metal leaching.
Applications and Future Outlook
- Biomass Valorization: This solvent-tuned strategy provides a practical route for converting HMF into high-value chemicals for pharmaceuticals, biofuels, and bioplastics.
- Reaction Control: The work highlights the decisive role of solvent–catalyst–substrate interactions in directing reaction pathways, offering a new lever for reaction design in biomass conversion.
- Industrial Potential: The simplicity of product control via solvent change makes this approach highly attractive for scalable, selective biomass upgrading.
This study not only advances our understanding of solvent effects in catalysis but also offers a promising and flexible strategy for the sustainable production of biomass-derived chemicals. Stay tuned for more innovations from Prof. Jianchun Jiang and Prof. Kui Wang’s teams!
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in