A single genomic deletion speeds up development in beetles, but makes them smaller
Published in Ecology & Evolution

Sometimes, departing postdocs can take a project from the lab with them to help start their new own lab. But this time, a supervisor and his team were allowed to continue working on a project that had been conceived and established by a postdoc who had left the lab. This project has now resulted in a publication in Nature Ecology & Evolution.
My postdoc Chris Jacobs began this project already during his PhD. We had been working for some time on immune defenses during embryonic development of the red flour beetle Tribolium castaneum (see e.g. Jacobs et al Elife 2014). But while investigating other insects, we suspected that these immune defenses in the egg trade off with embryonic developmental speed. In other words, insects that develop faster have less elaborate immune defenses, while extensive immune protection allows for longer development. Chris proposed to start selection lines for fast and slow embryonic development in the Tribolium beetle to examine this hypothesis in detail. I was not really in favour, since artificial selection takes time, other PhD projects had to be finished, and so on. I agreed on one condition: Chris had to think of a way to minimize the time spent on this courageous and risky project.

Together with his father Rien Jacobs, Chris built the machine shown above that he called the selectinator (see the machine in motion on YouTube ). Beetle eggs are placed on a mesh above a funnel, and freshly hatched larvae fall through to be collected in tubes on a rotating plate under the funnel. It was this machine that enabled us to generate Figure 1a and b of our Nature Ecology & Evolution paper which reveals a spectacular response of the populations to selection for fast or slow embryonic development. Chris continued selection when working as a postdoc, until funding made him switch from science to industry. In total, Chris had selected for four years (21 generations at 25 degrees) and counted more than 100.000 eggs. I was then allowed to continue working on the selection lines he left.
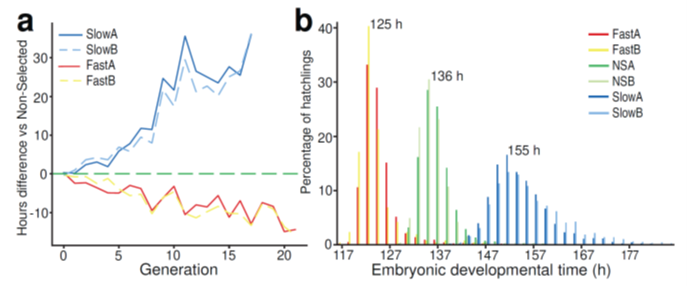
Although the trade-off with immune defenses continues to interest us, my group soon realized that developmental time of insects is currently a hot topic. Global warming can force insects to rapidly change their developmental time to remain in synchrony with their host plants or prey. The genetics of developmental time in insects turned out to be a fascinating topic in itself. Together with my next PhD student Shixiong Cheng, first author of the paper, we teamed up with Joost van den Heuvel in Wageningen to resequence all these selection lines and to investigate the genetic basis of developmental speed.
Joost identified two genomic regions under selection, in which Shixiong narrowed down the number of candidates genes by a qPCR and RNAi screen. This led Joost to discover a 222bp genomic deletion that had a high frequency in the fast lines, while this allele was nearly absent in the slow lines. This deletion is located in an enhancer of Cyp18a1, an enzyme that degrades ecdysone. Ecdysone is an important hormone regulating developmental transitions in insects, such as molting and pupation. What makes our paper stand out from other animal evolve-and-resequence studies, is that Shixiong could reconstruct this deletion in a laboratory strain of Tribolium using CRISPR/Cas9. This enabled us to study the exclusive effect of this allele, without disturbance from any other genetic variation. Shixiong found out how this deletion advances all ecdysone peaks in development, including an embryonic ecdysone peak that initiates dorsal closure.
The effect of this allele is that beetle development is sped up. However, speed comes with a cost. Fast beetles are lighter than beetles of the slow lines, and lay fewer eggs. I find it fascinating that a single genomic deletion can mediate such important life history decisions. Fast development may be useful if rapid adult dispersal is required when food sources are temporary. When the environment is more stable, though, it may pay off to develop longer, grow bigger, and lay more eggs. Such alleles may be common in natural insect populations and could facilitate rapid adaptation of insect developmental time to climate change. However, we do have to realize that such adaptation of developmental time is likely to have consequences for other insect life history traits, such as size or fecundity. A discovery that I would never have expected to do when Shixiong and I inherited selection lines from a departing postdoc….
Follow the Topic
-
Nature Ecology & Evolution

This journal is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Biodiversity and ecosystem functioning of global peatlands
Publishing Model: Hybrid
Deadline: Jul 27, 2026
Understanding species redistributions under global climate change
Publishing Model: Hybrid
Deadline: Jun 30, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in