A Strongly Coupled Cluster Heterostructure with Pt–N-Mo Bonding for Durable and Efficient H2 Evolution in Anion-Exchange Membrane Water Electrolyzers
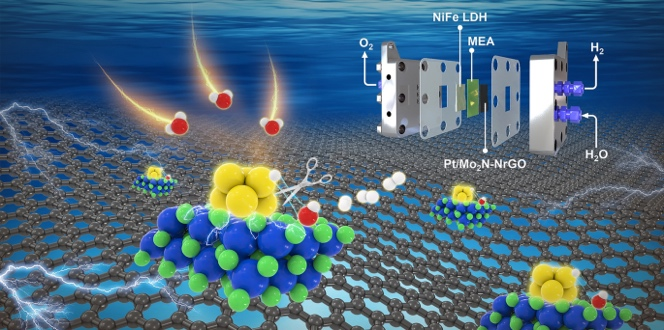
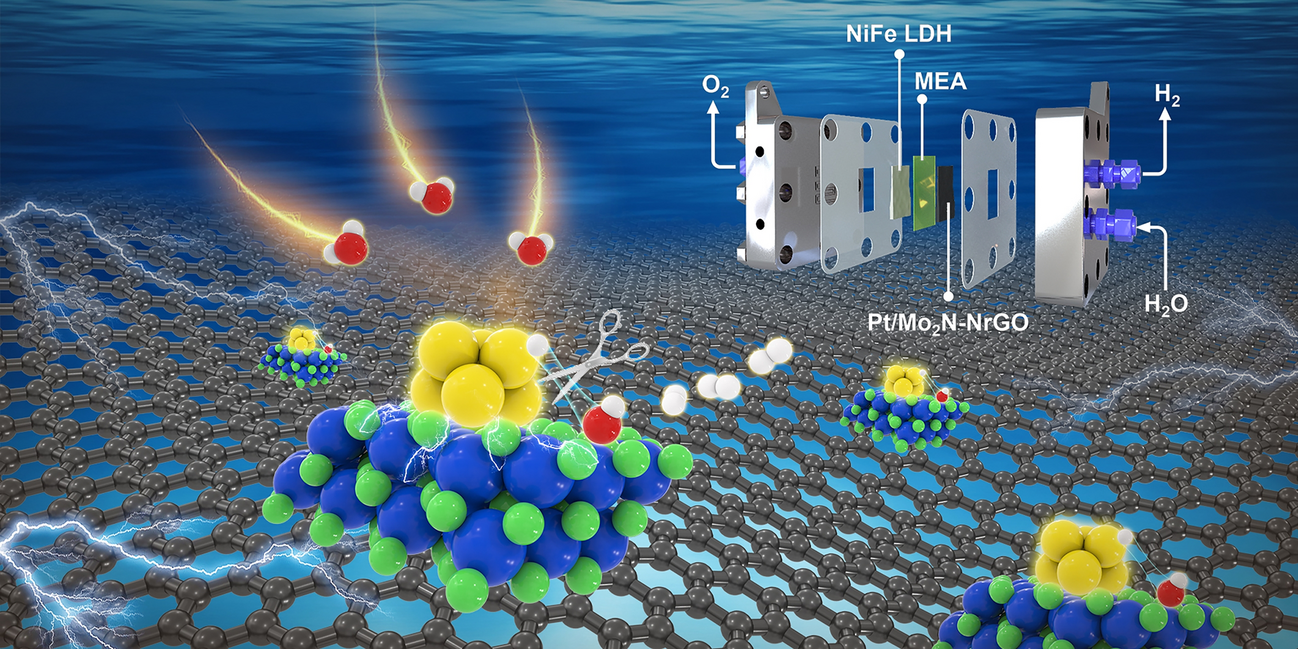
Alkaline water electrolyzers promise to unlock terawatt-scale green hydrogen, yet their Achilles’ heel remains the cathode: sluggish water-splitting kinetics, Pt nanoparticles that ripen into inert clumps, and price tags that still hover above the US DOE target of US$2 kg⁻¹. Now, a China–US collaboration led by Prof. Zhuangjun Fan (China University of Petroleum) and Prof. Zhenhai Wen (Fujian Institute of Research on the Structure of Matter) delivers a Pt/Mo2N cluster heterostructure on N-doped rGO that slashes overpotential, resists degradation for >500 h at 1.5 A cm-2, and drives the levelized cost of hydrogen down to US$2.02 kg-1—meeting the long-sought DOE benchmark.
Why This Catalyst Matters
- 11 mV at 10 mA cm-2– the lowest alkaline HER onset ever reported for a Pt-based system.
- 17.7 A mgPt-1 mass activity – 10× commercial Pt/C.
- 500-h durability at industrial 1.5 A cm-2 with <5 % potential drift.
- Techno-economic win – 1 MW plant model hits US$2.02 kg-1, inside DOE corridor.
Engineering the “Nano-Scissors” Interface
1. Anderson Clusters as Atomic Glue
- PtMo6 polyoxometalate precursor (1–2 nm) is nitrided at 800 °C to yield ~2 nm Pt/Mo2N clusters tightly stitched by Pt–N–Mo bonds (EXAFS at 1.56 Å).
- Charge transfer from Pt to Mo2N (Δ oxidation state +1.45) down-shifts the Pt d-band center (−2.73 eV), weakening H binding* while Mo sites anchor OH*—a molecular-scale “scissors” that cleaves H–OH in one stroke.
2. Operando Evidence
-
Live Raman at −0.25 V vs RHE tracks the Mo–OH (795 cm-1) and Pt–H (2328 cm-1) fingerprints simultaneously, revealing a bifunctional Volmer-Tafel pathway akin to acidic kinetics.
• DFT snapshots: Volmer barrier drops from 0.93 eV on Pt(111) to 0.36 eV on Pt/Mo2N; ΔG_H* moves to −0.07 eV—thermoneutral sweet-spot.
3. Device-Ready Performance
- AEMWE cell (Pt/Mo2N–NrGO || NiFe LDH) delivers 1.0 and 2.0 A cm-2 at only 1.66 V and 1.84 V at 80 °C—outperforming commercial Pt/C across the full temperature sweep.
- Energy efficiency peaks at 95 % (500 mA cm-2) and stays above 88 % at 1 A cm-2.
- Techno-economic model shows CAPEX falls 70 % from 100 to 2000 mA cm-2 while OPEX remains dominated by electricity; optimum sits at 560 mA cm-2 for the US$2.02 kg-1 LCOH.
Toward Gigawatt Deployment
- Roll-to-roll spray coatingalready demonstrated on 12 × 2 cm2 cells; scale-up roadmap targets m2-scale electrodes.
- Precursor is earth-abundant: Mo and N-rich urea cut noble-metal demand by 80 % versus state-of-art Pt/C.
- Next-gen roadmap: extend cluster strategy to seawater electrolysis and nitrate reduction—turning waste streams into hydrogen hubs.
Stay tuned as this cluster blueprint migrates from lab coin-cells to shipping-container electrolyzers, pushing green hydrogen past the tipping point.
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in