A sustainable thermochemical conversion of animal biomass to N-heterocycles
Published in Sustainability
Abstract
The production of high-valued organonitrogen chemicals, especially N-heterocycles, requires artificial N2 fixation accompanied by the consumption of fossil resources. To avoid the use of these energy- and resource-intensive processes, we develop a sustainable strategy to convert nitrogen-rich animal biomass into N-heterocycles through a thermochemical conversion process (TCP) under atmospheric pressure. A high percentage of N-heterocycles (87.51%) were obtained after the TCP of bovine skin due to the abundance of nitrogen-containing amino acids (e.g., glycine, proline, and L-hydroxyproline). Animal biomass with more diverse amino acid composition (e.g., muscles) yielded higher concentrations of amines/amides and nitriles after TCP. In addition, by introducing catalysts (KOH for pyrrole and Al2O3 for cyclo-Gly–Pro) to TCP, the production quantities of pyrrole and cyclo-Gly–Pro increased to 30.79 mg g−1 and 38.88 mg g−1, respectively. This approach can be used to convert the significant animal biomass waste generated annually from animal culls into valued organonitrogen chemicals while circumventing NH3-dependent and petrochemical-dependent synthesis routes.
Introduction
Organonitrogen chemicals are critical molecules for various industries including agriculture and medicine. N-heterocycles (such as pyrroles, pyridines, indoles, and cyclic dipeptides) are one of the most important classes of synthetic organonitrogen chemicals, accounting for more than 67% of compounds in the comprehensive medicinal chemistry database. Currently, N-heterocycles are synthesized in an energy- and resource-intensive two-step process where first N2 is fixed using high temperature and pressure (i.e., Haber–Bosch process), and the resultant NH3 is then reacted with carbon sources derived from petrochemicals. For example, the resultant NH3 reacted with petroleum derived furan to prepare pyrroles. Pyridines are often synthesized through condensation of aldehydes, ketones, or α, β-unsaturated carbonyl compounds with NH3 or NH3 derivatives. While, the high energy barrier of the N≡N bond (941 kJ mol−1) means that N2 fixation requires significant electricity consumption (1 to 3% of the world’s electricity) and fossil fuel consumption (3–5% of the world’s natural gas production). Therefore, efficient routes for synthesizing N-heterocycles and other organonitrogen chemicals need to be developed to circumvent the current dependence on artificial N2 fixation, harsh reaction conditions, fossil resources, and multiple energy-intensive synthesis steps.
Along with the ardent desire to transform the chemical industry into a greener and more sustainable manner, it will be significant to explore alternative routes to access organonitrogen chemicals from renewable sources. Recently, plant biomass (e.g., cellulose, hemicelluloses, and lignin) becomes one of the strategies to synthesize high-valued organonitrogen chemicals from renewable resources without the need for carbon from fossil resources. However, extrinsic nitrogen source is still required due to the relative absence of nitrogen in plant tissue (0.3 to 5 wt.% of dry weight). For instance, after being impregnated with 10% urea, wood chips could transform into pyrolysis oil with large content of organonitrogen chemicals. Ammonia can also react with the cracked bonds (C–C, C–O, C=O, C–H and/or O–H) of cellulose during the thermal treatment to obtain a large number of diverse nitrogen-containing compounds. Moreover, with the help of ammonia, furfural (a platform chemicals from hemicellulose) could be a substrate for the production of N-heterocycles through a single-step decarbonylation-amination reaction in a catalytic system. Generally, the key chemistry of the above strategies is based on the transformation of oxygen-containing groups of these starting materials into nitrogen-containing molecules with the involvement of ammonia or its derivatives derived from artificial N2 fixation. Therefore, the need of fossil resources, harsh reaction conditions, and multiple energy-intensive synthesis steps still significantly hampered the further development of these plant biomass-based production of organonitrogen chemicals.
Nitrogen-rich biomass resource utilization can expand the boundaries of biorefinery and product diversity. The natural nitrogen cycle in the ecosystem begins with N2 fixation (e.g., diazotrophs), after which nitrogen migrates up the food chain and finally accumulates mainly in the form of amino acids (Fig. 1a). Therefore, animal biomass often contains higher nitrogen (> 12 wt.% of dry weight) than that of plant biomass, and this abundant nitrogen is primarily in the form of proteins. This accumulation of nitrogen in animals potentially offers a route towards the one-step conversion of biomass into organonitrogen chemicals. Currently, no previous studies have been reported focusing on the potential use of animal biomass in synthesizing high-valued organonitrogen chemicals. Composed of amino acids, animal tissues require relatively less energy to decompose compared with chitin, or other nitrogen-rich biomass, which needs complex or harmful pre-treatments to break down the recalcitrant structures due to the high crystallization and widespread hydrogen-bond networks. Moreover, from a sustainable and ethical perspective, the number of the top five species of livestock is ever expanding and has reached 2.77 × 1010 in 2017 globally, and about 1.11 × 109 animal carcasses need to be disposed annually due to the general mortality of livestock (3–5%), not to mention carcasses related to culls. This large number of waste animal biomass threatens public health safety and can cause severe environmental problems without proper and prompt disposal.
.jpg)
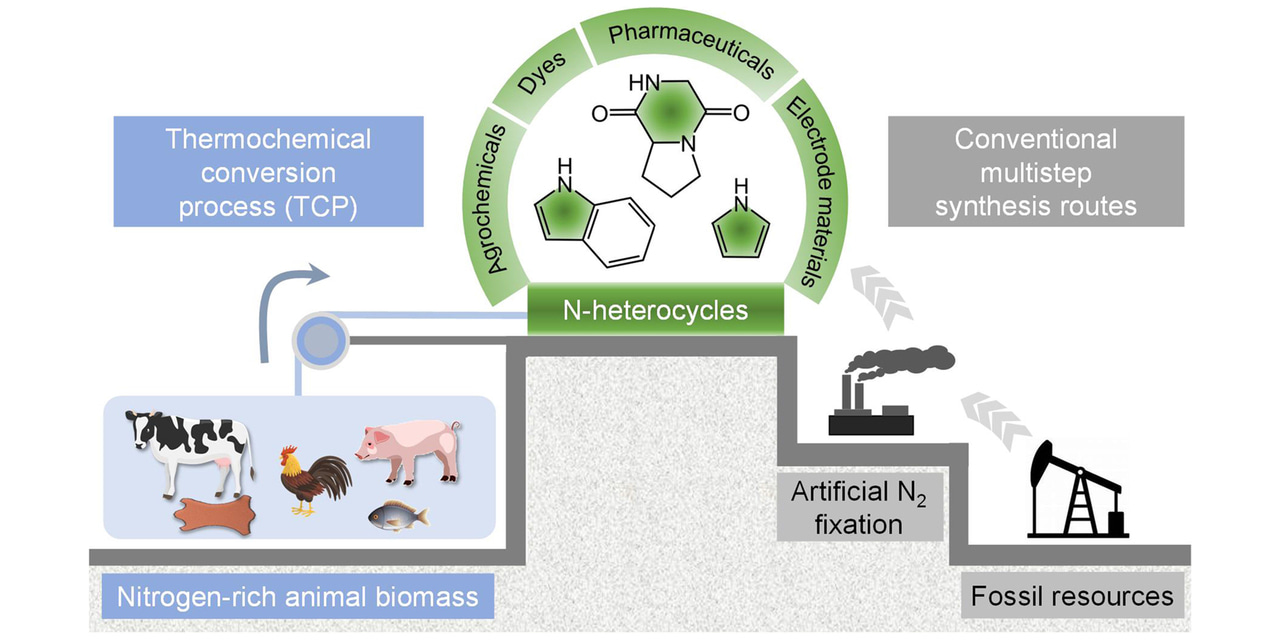
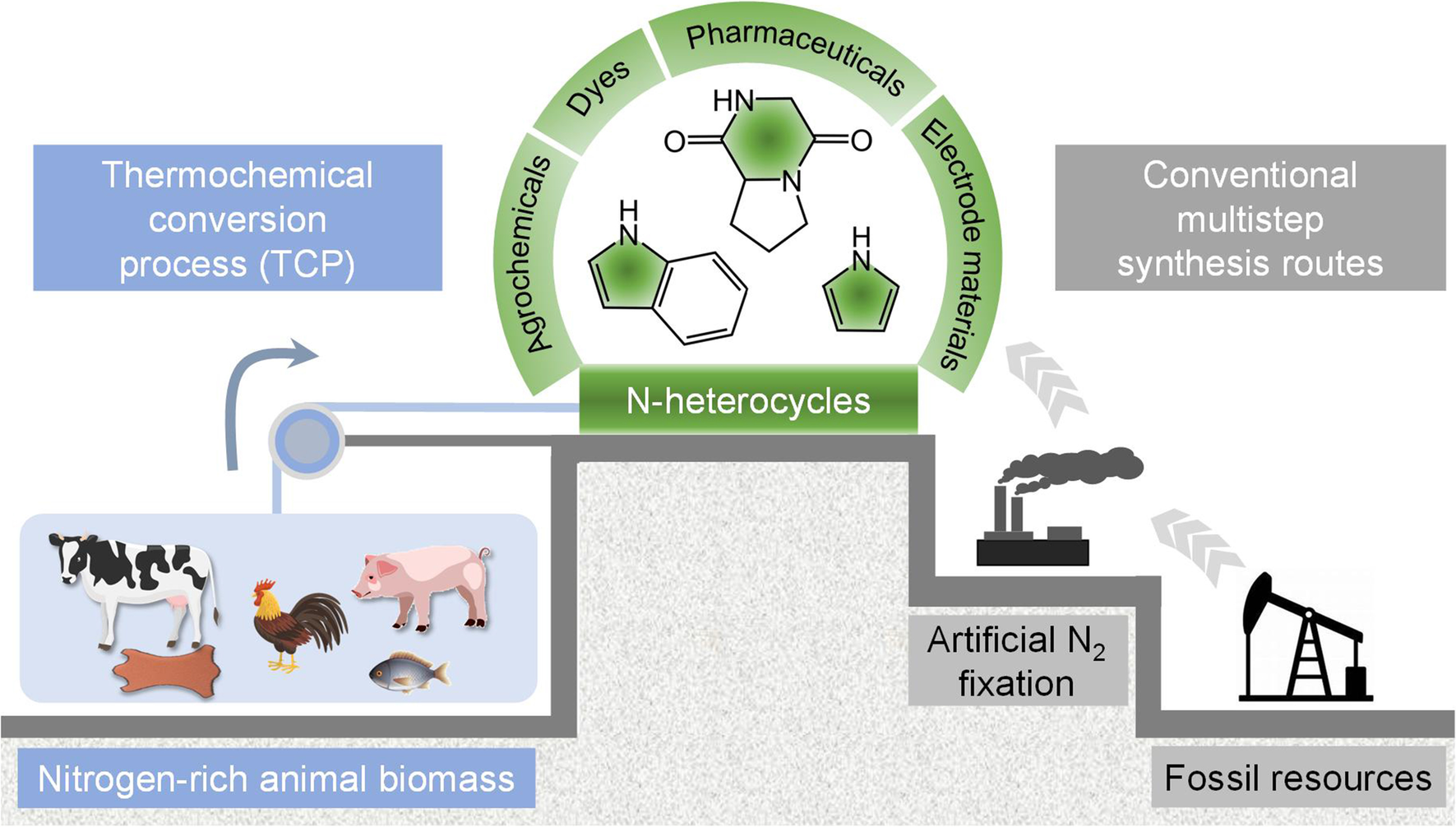
Fig.1 Comparison between the conventional two-step route and the one-step animal biomass-dependent TCP route to generate N-heterocycles. a Schematic illustration of organonitrogen synthesis using TCP compared with the traditional chemical synthesis based on artificial N2 fixation and fossil resources conversion (based on the Haber–Bosch process). b Schematic of the internal structure of the TCP device with the controlled temperature from 300 to 800 °C under atmospheric pressure. c Molecular structures and industrial applications of representative obtained N-heterocycles. d Optical photographic image of the installation of an actual TCP device
Herein, this investigation develop a viable one-step strategy to produce N-heterocycles from animal biomass obtainable from culls or processing plants (i.e., bovine skin, bovine muscle, chicken muscle, porcine muscle, and fish muscle) using thermochemical conversion process (referred to as TCP) under atmospheric pressure. Compared with the traditional chemical synthesis involved in artificial N2 fixation and refinement of fossil resources, TCP presents as a more convenient and straightforward way to obtain N-heterocycles (Fig. 1b–d), during which the organic intermediates of nitrogen-rich animal biomass are largely decomposed and restructured during the thermochemical process (Additional file 1: Figs. S2–6). Our results show that the bio-oil produced from bovine skin after TCP has a high content of N-heterocycles (87.51% in all nitrogenous products), while other types of animal biomass result in a more uniform distribution of N-heterocycles, amines/amides, and nitriles. Mechanistic studies provide insight into the formation mechanisms of different N-heterocycles and how the presence and concentration of these compounds after TCP correlate with the distribution of amino acids in the different biomass sources. For example, cyclo-Gly–Pro could be easily generated after the dehydration condensation of glycine (Gly) and proline (Pro) in bovine skin. These findings demonstrate that the conventional two-step synthesis routes currently used for synthesizing organonitrogen chemicals can be completely circumvented through the further development of this sustainable, one-step thermochemical conversion strategy of animal biomass.
Follow the Topic
-
Collagen and Leather

This journal focuses on new findings and scientific advances in all aspects of collagen and leather, aiming to provide a platform for both scientists and engineers to address and discuss the theoretical and practical problems encountered in leather manufacture and novel utilizations of collagen.
What are SDG Topics?
An introduction to Sustainable Development Goals (SDGs) Topics and their role in highlighting sustainable development research.
Continue reading announcement




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in