Adhesive bandage for quick SARS-CoV-2 antibody detection
Published in Bioengineering & Biotechnology

Adhesive bandage point-of-care test
Rapid testing allows for prompt isolation and treatment of infected individuals. Immunoglobulin M (IgM) and immunoglobulin G (IgG) are natural “first-line-of-defense” antibodies against viral infections1. These antibodies -produced in blood by our immune system- can serve as valuable biomarkers to identify infected individuals and monitor the spread of pandemics2. The developed adhesive bandage (Figure 1) detects the IgM and IgG antibodies specific to SARS-CoV-2 virus, in a quick and sensitive manner.
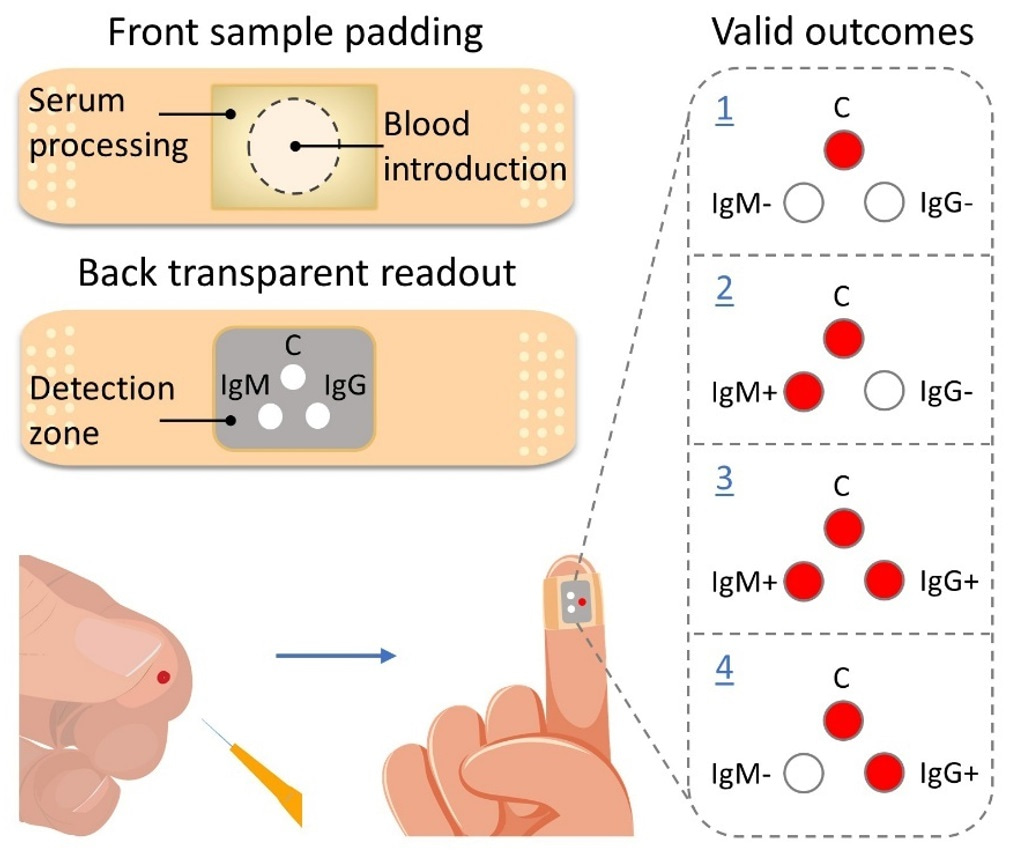 Figure 1. Schematic of the adhesive bandage test for detection and screening of SARS-CoV-2 IgM and IgG antibodies with valid outcomes confirming 1: negative (IgM−/IgG−) or 2-4: positive (IgM+/IgG−, IgM+/IgG+, IgM−/IgG+, respectively) for SARS‐CoV‐2 infection. C represents a positive control spot for the valid outcomes.
Figure 1. Schematic of the adhesive bandage test for detection and screening of SARS-CoV-2 IgM and IgG antibodies with valid outcomes confirming 1: negative (IgM−/IgG−) or 2-4: positive (IgM+/IgG−, IgM+/IgG+, IgM−/IgG+, respectively) for SARS‐CoV‐2 infection. C represents a positive control spot for the valid outcomes.
Capture and Detection of SARS-CoV-2 IgM and IgG Antibodies
The adhesive bandage test captures and detects SARS-CoV-2 IgM and IgG antibodies using gold nanoparticles. With a diameter of one billionth of a meter, these tiny nanotechnology-based particles are bioactivated with unique SARS-CoV-2 surface proteins (antigens) (Figure 2a). The antigens act as "keys" to “lock” the IgM and IgG antibodies with specificity and sensitivity similar to traditional ELISA tests commonly used in clinical settings3. This interaction results in visible color changes that can be observed and interpreted by the naked eye (Figure 2b). Paralleled with standard pregnancy tests4, the adhesive bandage test requires only a tiny drop (pinprick) of blood to detect the antibodies, making it minimally invasive with the use of lancets. Combined, this advanced approach makes the adhesive bandage test highly promising for widespread point-of-care and point-of-use testing.
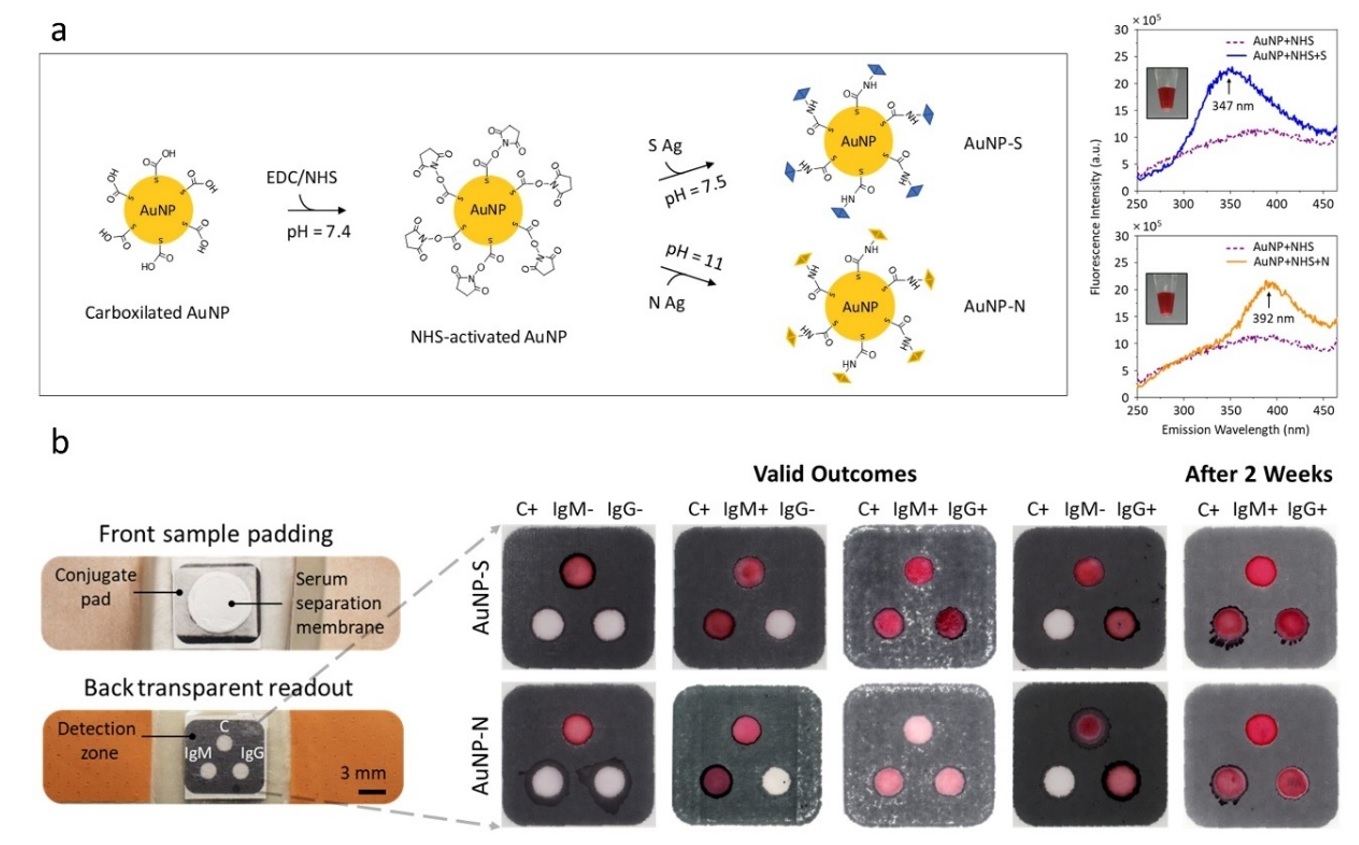 Figure 2. a Bioactivation of the gold nanoparticles (AuNPs) (40 nm diameter) with SARS-CoV-2 specific spike (S) and nucleocapsid (N) antigens in buffer conditions and their characterization using fluorescence spectroscopy. Left panel: Schematic illustrates the steps involved in their bioactivation using EDC/NHS chemistry. Right panel: Intrinsic fluorescence measurements of the S and N antigens (maximum emissions pointed by arrows), with excitation at 240 nm, further validates their successful chemisorption to NHS-activated AuNPs. Insets: Micrographs show the stability of bioactivated AuNPs under optimized conditions, where the reddish color represents monodispersed AuNPs. b Micrographs show the developed adhesive bandage test (left panel) with tested four different antibody capture and detection conditions (right panel) that mimic the respective schematic in Figure 1. The conjugate pad, as the middle bioactive layer, was chemically modified5 to contain 3 hydrophilic spots to match the spots in the back readout (detection zone). The bandage demonstrated excellent reproducibility for long-term (2 weeks) storage stability.
Figure 2. a Bioactivation of the gold nanoparticles (AuNPs) (40 nm diameter) with SARS-CoV-2 specific spike (S) and nucleocapsid (N) antigens in buffer conditions and their characterization using fluorescence spectroscopy. Left panel: Schematic illustrates the steps involved in their bioactivation using EDC/NHS chemistry. Right panel: Intrinsic fluorescence measurements of the S and N antigens (maximum emissions pointed by arrows), with excitation at 240 nm, further validates their successful chemisorption to NHS-activated AuNPs. Insets: Micrographs show the stability of bioactivated AuNPs under optimized conditions, where the reddish color represents monodispersed AuNPs. b Micrographs show the developed adhesive bandage test (left panel) with tested four different antibody capture and detection conditions (right panel) that mimic the respective schematic in Figure 1. The conjugate pad, as the middle bioactive layer, was chemically modified5 to contain 3 hydrophilic spots to match the spots in the back readout (detection zone). The bandage demonstrated excellent reproducibility for long-term (2 weeks) storage stability.
Advantages of the Adhesive Bandage Test
What sets the adhesive bandage test apart is its simplicity and convenient usage in pandemic situations, thus offering a practical solution for rapid antibody detection at homes, public facilities, and rural regions. The bandage comes at a low cost and is disposable, thus holding promise for large-scale usage and real-time screening during pandemics. Importantly, the bandage can be potentially integrated with biodegradable porous microneedles6 in the future, enabling efficient finger pricking via in situ puncturing. This would eliminate the need for lancets, thus reducing the extra step for obtaining a blood sample and minimizing the risks of infection at the puncture site.
Beyond SARS-CoV-2
The adhesive bandage test holds great promise as a versatile point-of-care device for future pandemics since it can be easily and quickly modified to detect other viral infections by using antigens specific to emerging viruses.
Preventing Future Pandemics
Real-time screening using tests like the adhesive bandage test can play a crucial role in preventing future pandemics by enabling early detection. Similar to how regular bandages are used to cover and protect wounds, the adhesive bandage will be applied to a fingertip to detect antibodies in a pinprick of blood. In the future, if the large-scale usage of the developed adhesive bandage test is combined with smart phone readouts and dedicated mobile apps, it opens up possibilities for generating location-based heat maps by local governments and health authorities to allow for the early identification of infected regions, including patients with no symptoms. By promptly isolating these regions and treating patients, the spread of the virus can be significantly contained, preventing it from reaching a larger population.
Conclusion
The adhesive bandage test represents an advancement in point-of-care and point-of-use testing. By bringing the diagnosis of viral infections to people's fingertips, this innovative approach offers accessibility, convenience, and cost-effectiveness, making it suitable for large-scale usage. The bandage can also be easily adjusted and optimized to detect different viral infections as they arise by simply selecting appropriate antigens related to pandemics.
References
1 Price C. P. Point-of-care testing - Impact on medical outcomes. Clin Lab Med 21, 285-303 (2001).
2 Long Q. X. et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 26, 845-848 (2020).
3 Chernesky M. A. & Mahony J. B. Detection of viral antigens, particles, and early antibodies in diagnosis. Yale J Biol Med 57, 757-776 (1984).
4 Bahadir E. B. & Sezginturk M. K. Lateral flow assays: Principles, designs and labels. Trends Anal Chem 82, 286-306 (2016).
5 Samara B. et al. Cryopreservable arrays of paper-based 3D tumor models for high throughput drug screening. Lab Chip 21, 844-854 (2021).
6 Bao L. L. et al. Anti-SARS-CoV-2 IgM/IgG antibodies detection using a patch sensor containing porous microneedles and a paper-based immunoassay. Sci Rep 12, 10693 (2022).
Follow the Topic
-
Microsystems & Nanoengineering

This journal, with a target for a high-end journal for years to come, seeks to promote research on all aspects of microsystems and nanoengineering from fundamental to applied research.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in