An alternative access to chiral spiro-tetrahydrofurans
Published in Chemistry
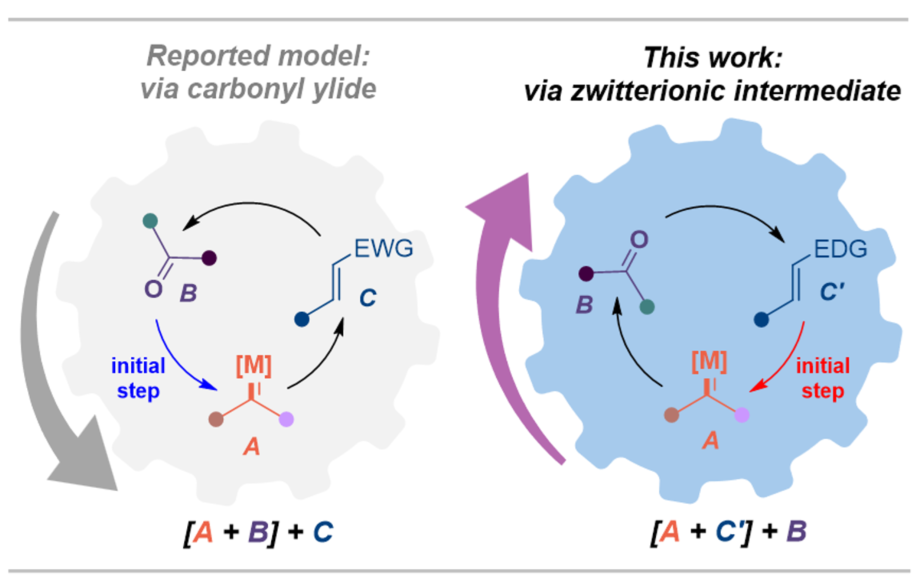
An asymmetric formal [1+2+2]-cycloaddition of readily accessible diazoamides with enamines and carbonyl compounds has been disclosed. This cascade reaction involves a 1,3-dipolar cycloaddition of ketones/aldehydes with the key zwitterionic intermediate that generated in situ through metal carbene transfer to nucleophilic enamines, which provides a direct access to spiro-tetrahydrofurans containing four consecutive stereocenters in high yields and excellent enantioselectivity. Notably, this cycloaddition reaction offers a complementary protocol for the stereoselective construction of chiral tetrahydrofurans, which are regioisomers of those formed by traditional [3+2]-cycloadditions, such as [3+2]-cycloaddition of carbonyl ylide with alkenes , or [3+2]-annulation using donor-acceptor cyclopropanes as C3 building blocks.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in