An implantable optical nanosensor
Published in Bioengineering & Biotechnology

In Dr. Heller’s lab, I became interested in the clinical application of a property of carbon nanotubes that I wasn’t even aware existed: an intrinsic fluorescence in the near-infrared spectral region [1]. This fluorescence does not photobleach, as most fluorescent molecules do, and the intensity and wavelength of the fluorescence is very sensitive to molecules near the nanotube’s surface. And certainly interesting from a biomedical engineering standpoint, this wavelength of light is ideal for maximally penetrating tissue, opening exciting possibilities for measurements through living tissue in humans, such as an implantable sensor [2,3].
Over the past few years, short strands of DNA [4] and RNA [5] that can be found in different biological fluids have been intensely studied as promising biomarkers, especially for diseases such as cancer. We identified microRNA (miRNA) as particularly interesting for this type of application [5]. With this in mind, we intended to design a sensor that would reliably produce a signal upon binding a target oligonucleotide and retain versatility to be programmed to detect any sequence. With the diverse expertise of the Heller lab team, we built spectroscopic instruments and analysis schemes for rapid testing, and I was eventually able to design a sensor that would respond specifically to a target oligonucleotide sequence.
Upon binding a miRNA, the sensor responded specifically to the target sequence via a small blue shift in the nanotube emission band. Although we were happy with the selectivity of the sensor, we were disappointed with the relatively small optical response that the sensor produced upon binding. So we sought to better understand how changes on the nanotube surface led to the change in emission. Working with great collaborators at Lehigh University, Dr. Jeetain Mittal and his team, we found that molecular dynamics simulations could help us to understand the factors at play.
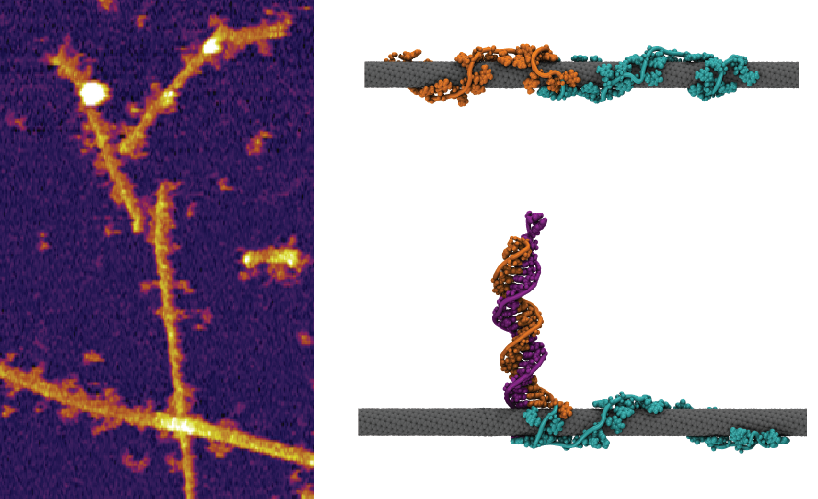
Figure 1: (Left) Atomic force microscope image of the nanotube sensor bound to target oligonucleotide. (Right) Snapshots from our molecular dynamics simulation of the hybridized sensor complex.
We knew that the removal of water from the nanotube’s surface should result in a blue shift in the nanotube emission, but the simulations showed that hybridization would remove DNA and allow more water to access the nanotube surface (Figure 1), resulting in the opposite condition. We found, however, that hybridization also removed a large amount of anionic charge from the nanotube surface, due to the phosphate groups on the DNA backbone, which was the likely reason for the small overall blue-shifting response. But the simulations also highlighted an opportunity — the opening up of substantial free space on the nanotube’s surface upon hybridization.
Armed with the insight gained through the simulations, we hypothesized that we could improve the sensing response via addition of an amphiphilic molecule to bind to the newly freed nanotube surface and remove water, and thereby enhance the blue shift. Upon screening candidates, we found a surfactant [6] that could be used to achieve a large enhancement in sensor response to hybridization.
The pace of the project rapidly picked up and we moved forward in characterizing the system. We set out to assess the clinical applicability of the technology both for in vitro measurements in biofluids and as an implantable sensor that can be interrogated non-invasively. In two common biofluids, serum and urine, we were able to directly detect oligonucleotides without purification and with minimal pre-treatment. We then implanted the nanotubes, protected by a semipermeable membrane, into a mouse’s belly cavity, and then we injected target miRNA. By simply shining light at the mouse’s belly and recording the emitted light (Fig. 2, left), we found that the wavelength of the nanotubes changed in response to the RNA. We found that we could detect this molecule in live mice by using nothing more than light; it was as easy as reading a barcode.
We envision a future where a small device containing the nanotubes can be implanted (or injected) in a patient and continuously monitored with an external light source, allowing for the early detection of diseases. The light source and detector could perhaps be worn [7,8] (Fig. 2, right), and the data could be wirelessly transmitted and analyzed [9].
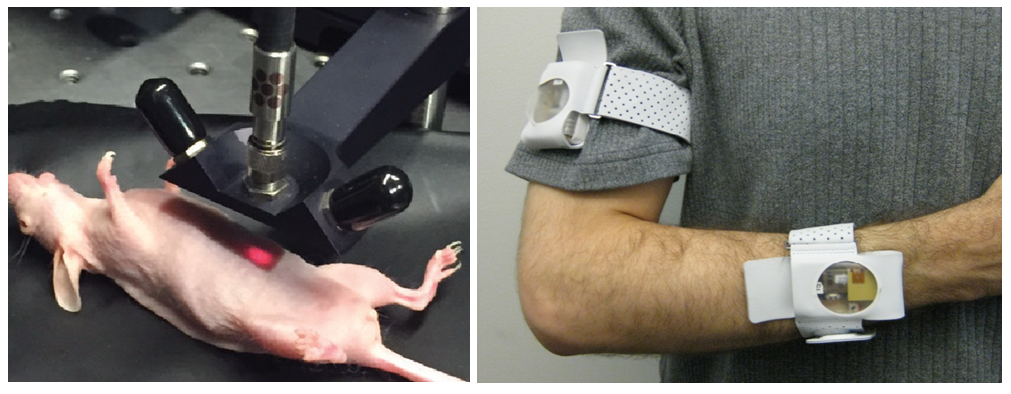
Figure 2: (Left) Non-invasive miRNA measurement via the implanted sensor upon excitation with red light. (Right) Example of a wearable medical device for continuous monitoring of motion (image taken from Mukhopadhyay [8]).
Our paper:
Harvey, J. D. et al. A carbon
nanotube reporter of microRNA hybridization events A carbonnanotube reporter of microRNA hybridization events in vivo. Nat.
Biomed. Eng. 1, 0041 (2017).
References
1. O'Connell, M.J., Bachilo, S.M., Huffman, C.B. et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 297, 593-596 (2002).
2. Barone, P.W., Baik, S., Heller, D.A. & Strano, M.S. Near-infrared optical sensors based on single-walled carbon nanotubes. Nature materials 4, 86-92 (2005).
3. Iverson, N.M., Barone, P.W., Shandell, M. et al. In vivo biosensing via tissue-localizable near-infrared-fluorescent single-walled carbon nanotubes. Nature nanotechnology 8, 873-880 (2013).
4. Dawson, S.J., Tsui, D.W., Murtaza, M. et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. The New England journal of medicine 368, 1199-1209 (2013).
5. Mitchell, P.S., Parkin, R.K., Kroh, E.M. et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proceedings of the National Academy of Sciences of the United States of America 105, 10513-10518 (2008).
6. Haggenmueller, R., Rahatekar, S.S., Fagan, J.A. et al. Comparison of the quality of aqueous dispersions of single wall carbon nanotubes using surfactants and biomolecules. Langmuir : the ACS journal of surfaces and colloids 24, 5070-5078 (2008).
7. Yilmaz, T., Foster, R. & Hao, Y. Detecting Vital Signs with Wearable Wireless Sensors. Sensors 10, 10837-10862 (2010).
8. Mukhopadhyay, S.C. Wearable Electronics Sensors For Safe and Healthy Living. Smart Sensors, Measurement and Instrumentation 15, V-Vii (2015).
9. Boulos, M.N.K., Wheeler, S., Tavares, C. & Jones, R. How smartphones are changing the face of mobile and participatory healthcare: an overview, with example from eCAALYX. Biomed Eng Online 10 (2011).





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in