An Integrated Approach for Representing Knowledge on the Potential of Drugs to Cause Acute Kidney Injury
Published in Biomedical Research, General & Internal Medicine, and Pharmacy & Pharmacology
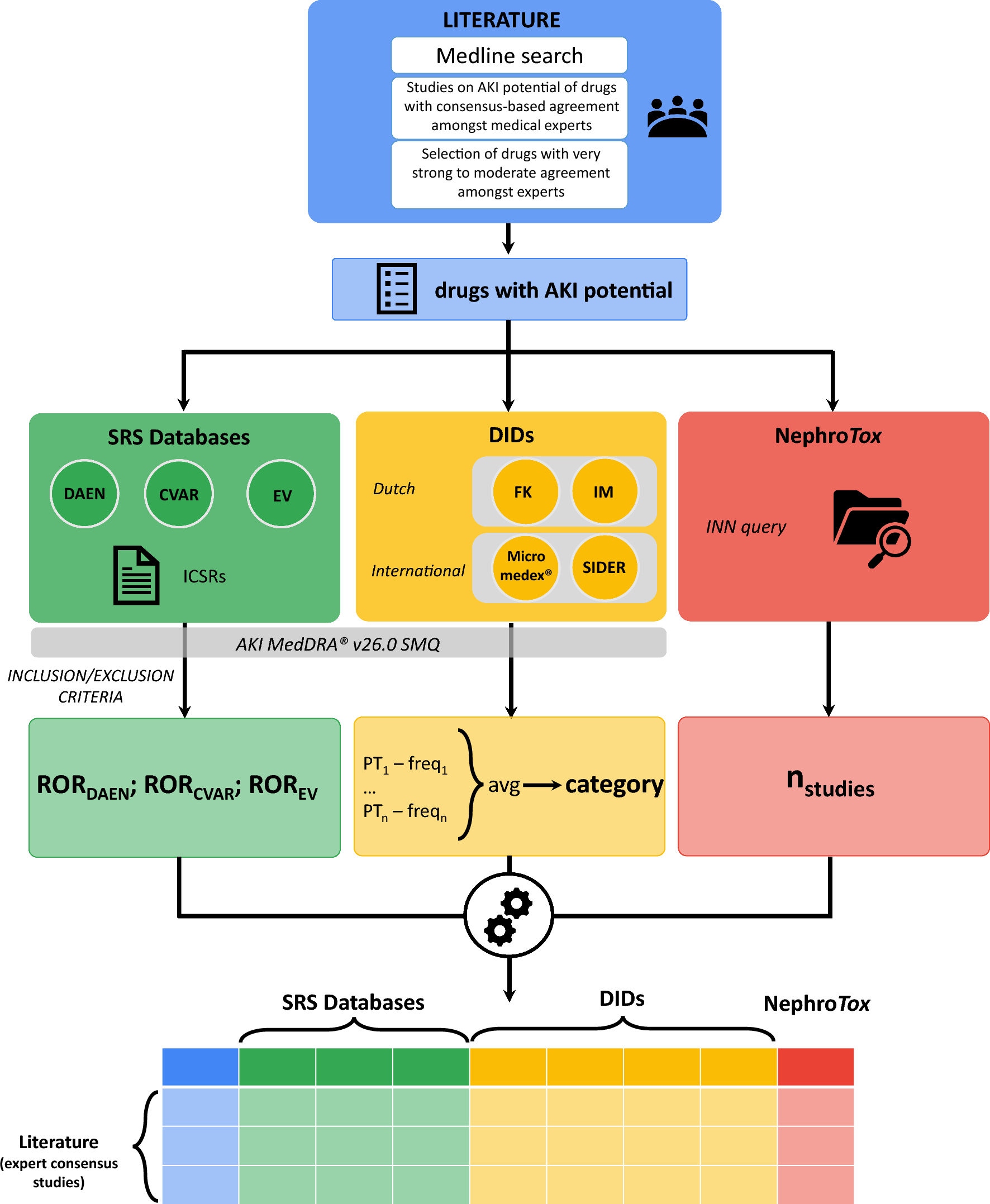
Introduction. AKI occurs in up to 20% of hospital admissions and one of the leading causes are drugs that patients are exposed to during treatment, with certain pharmacological classes posing higher risks. Past research has often been limited by a fragmented approach, relying on individual studies or expert consensus lists without integrating data from pharmacovigilance databases, literature reviews, and clinical observations. The limitations of these studies include variability in data sources and methodological differences. To address this, we combined these multiple sources of information—expert consensus, spontaneous report system (SRS) databases, drug information databases (DIDs), and NephroTox website—to develop a more comprehensive understanding of drugs with AKI potential.
Results.
Study selection for assessment of drugs with AKI potential. A total of 5,935 studies were initially screened, with four key expert consensus studies being included based on predefined inclusion criteria. We extracted the drugs associated with AKI from these studies, and expert consensus was used to classify drugs according to their potential for causing AKI. The drugs identified with AKI potential were divided into several levels of agreement: limited agreement (n=131), moderate agreement (n=36), strong agreement (n=17), very strong agreement (n=16). Drugs which gathered moderate to very strong agreement between experts (n=63) were selected for further analyses, with antibacterials (17.5%), antivirals (15.9%), and NSAIDs (11.1%) being the most frequent pharmacological classes.
Disproportionality Analysis. SRS databases, which include data from Australia’s DAEN, the Canadian CVAR, and the European EV, revealed strong signals (reporting odds ratio) for AKI-related adverse drug events (ADEs) in certain classes like antivirals, antibacterials, NSAIDs, and immunosuppressants. Renin-angiotensin-aldosterone system (RAAS) inhibitors and diuretics showed strong signals across SRS databases, but this is likely due to haemodynamic changes.
AKI Mechanisms. Among the drugs with AKI potential, the majority caused damage without kidney dysfunction (n=41, 65.1%), while only a minority (n=9, 14.3%) showed dysfunction without damage.
AKI Frequencies from DIDs. We also examined AKI-related ADE frequencies across four DIDs, including Micromedex®, SIDER and the Dutch drug databases Farmacotherapeutisch Kompass and Informatorium Medicamentorum from KNMP. Across the four DIDs, antibacterial and antiviral drugs were associated with the highest frequencies of AKI-related ADEs, while NSAIDs exhibited significant variability. While Micromedex® showed that most drugs with AKI potential had frequent to very frequent AKI-related ADEs, the Dutch DIDs indicated that these events were rare or very rare. This inconsistency highlights the challenges of using different data sources and the importance of consulting multiple databases to assess AKI risks.
NephroTox website. All 63 drugs identified with moderate to very strong expert agreement had at least two published studies on adverse kidney events, with antibacterials and antineoplastic agents having the largest number of studies. NSAIDs and RAAS inhibitors showed greater variability in NephroTox, indicating that further research is needed to assess their AKI risks consistently.
Knowledge Integration from Multiple Sources. When integrating data from the four sources (expert consensus, SRS databases, DIDs, and NephroTox), we identified patterns across drug classes. Drugs with very strong or strong agreement among experts tended to show consistent signals of AKI potential in SRS databases, while drugs with moderate agreement showed higher variability. Drugs in the moderate agreement category also exhibited more missing or unreported AKI-related ADE frequencies in DIDs compared to those with stronger expert agreement.
A comprehensive approach combining expert opinion with real-world data from pharmacovigilance databases and drug information sources offers a more complete picture of drug-induced AKI risks. However, challenges remain, such as variability in ADE reporting, differences in content refresh rates across DIDs, and discrepancies between signals in SRS databases and the number of safety studies carried out as identified by NephroTox.
However, the study also has limitations. For example, we relied on existing expert consensus lists, which may have missed certain drugs with known AKI potential. The focus on drugs approved in the Netherlands may limit generalisability to other countries, though many of the identified drugs are common globally. Additionally, the study did not include causality assessments for AKI-related adverse events, and the signals from disproportionality analyses are merely associational.
Conclusion. Our study provides a valuable resource for assessing the AKI potential of drugs by integrating expert consensus, pharmacovigilance data, drug information databases, and published studies. This multi-source approach represents a significant step forward in advancing the understanding of DAKI, informing clinical decision-making, and improving kidney safety in prescribing practices. The authors encourage continued efforts to refine this resource and further investigate drugs with AKI potential, particularly those with limited or inconsistent evidence.
Future Research Directions. Future research should further investigate the 131 drugs with limited agreement, especially focussing on antitumor agents and other drug classes not thoroughly assessed in this study. We also call for improvements in data collection and standardisation across databases to enable better integration of evidence and more robust causality assessments.
Follow the Topic
-
Drug Safety

This journal advances the rational use of pharmacotherapy by publishing reviews and original research articles offering guidance for safe and effective drug utilization and prescribing.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in