Announcing ISRCTN’s new Transparency Tracker and Badges
Published in Biomedical Research and Public Health
We’re excited to present the release of the ISRCTN clinical study registry’s new and improved Transparency Tracker and Badges.
Curious about what these tools are and why they’re useful in the clinical landscape? We’ve put together a handy guide below.
Why is transparency so vital in clinical studies?
The Health Research Authority’s Make It Public campaign demonstrates that research transparency is essential for fostering trust and accountability with patients and the public, avoiding duplication of efforts, and to improve the quality of studies being carried out.

Transparency also tackles issues like publication bias and selective reporting, while making ongoing studies more visible boosts awareness and opens up new opportunities for collaboration.
How do you measure transparency in clinical studies?
Well, that’s a bit of a ‘how long is a piece of string?’ question. You could take measuring transparency in so many different directions, depending on whether you’re looking from the perspective of a funder, a patient or a researcher.
A great starting point is the WHO International Standards for Clinical Trials Registries, but we wanted to make the process a little bit more visual than flicking through a 49 page PDF each time you want to consider how transparently a study is being carried out.
So we’ve distilled this down to six key elements that are measurable for every clinical study. These are:
- Prospective or retrospective registration
- Protocol sharing
- Statistical analysis plan sharing
- Results sharing
- Individual participant data sharing
- Updating a study within the last year
What does the Transparency Tracker do?
The Transparency Tracker looks at these six elements at a whole registry level, displaying a dashboard of graphs which show the percentage of studies which conform to each WHO standard.
For example, what proportion of interventional studies registered on ISRCTN are prospectively registered, before the first participant has been enrolled?
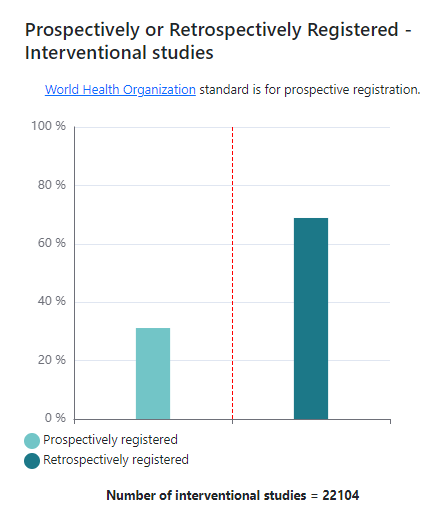
And what proportion of interventional studies have had a results summary or a link to a published journal article added to their record on the registry?
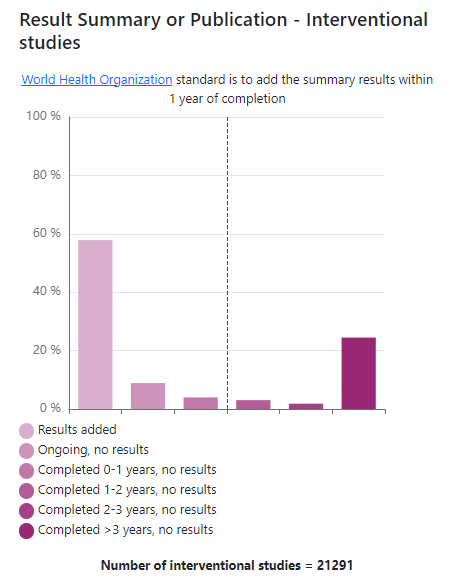
The Transparency Tracker is connected up to our advanced search, so it’s possible to view the dashboard at a filtered level, for example to look at studies linked to specific diseases, funders or interventions. You can also navigate backwards to a list of relevant studies by clicking on each interactive bar of a bar chart.
What are the Transparency Badges for?
The Transparency Badges measure the same standards as the Tracker, but at the level of an individual study. Each clinical study record on ISRCTN has six badges – one for each of the factors we measure based on the WHO’s standards. These will be shaded in blue if the criteria has been achieved, or left empty if this isn’t yet the case.
For example, the badges from the study below show that it was prospectively registered, updated within the last year, and has had a protocol and statistical analysis plan added, but is still waiting for the results and individual participant data to be added when the study concludes:

The launch of the new Transparency Tracker and Badges mark a significant step forward in being able to support and encourage transparent clinical studies on ISRCTN. We hope these tools not only act as clear, visual representations of these six key transparency standards, but also empower our users to consider and assess these for themselves.
Don't forget to take a look at the Transparency Tracker here, or at the badges on any of the studies registered with ISRCTN here!
Follow the Topic
-
ISRCTN: The UK’s Clinical Study Registry

A primary clinical trial registry recognised by WHO and ICMJE that accepts studies involving human subjects or populations with outcome measures assessing effects on human health and well-being, including studies in healthcare, social care, education, workplace safety and economic development.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in