Antimony Selenosulfide: An Emerging Solar Material with 10% Efficiency
Published in Materials

Sb2(S,Se)3 is a kind of emerging solar material with simple composition, high stability as well as abundant elemental storage. The crystal structure is composed of (Sb4S(e)6)n ribbons (Fig. 1a). Sulfur and selenium are completely miscible, which enables the band gap tunable in 1.1-1.7 eV range, falling into the optimum band gap estimated by Shockley-Queisser limit.
A recent article in Nature Energy reported a hydrothermal reactive deposition method (Fig. 1b) for the synthesis of Sb2(S,Se)3 film for solar cells (Fig. 1c) and made a breakthrough in the power conversion efficiency towards 10%.

It has been noted that there are several kinds of methods applied in the synthesis of alloy type Sb2(S,Se)3, which enables the device efficiency around 7%. The hydrothermal deposition seems unique to generate high quality Sb2(S,Se)3 film. Here I analyze the reasons from both the materials properties and deposition characteristics.

Firstly, the hydrothermal deposition method using water as solvent would not cause the generation of carbon residues, which is usually a problem in the “spin-coating” method with organic solvent. Furthermore, it can generate highly soluable precursor materials, rendering the formation of homogenous solution. Our experience indicated that the solubility is important. If there are insoluble precursors, the reactive deposition would generate local inhomogeneity, which further generate trap state and block the carrier transport.
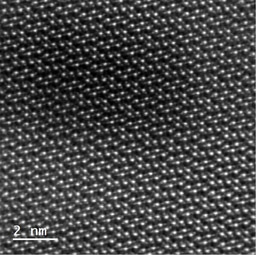
The hydrothermal environment provides required driving force for generating energetic ions to be deposited onto the substrate. In addition, according to our estimation, the concentration of reactive ions is several orders of magnitude higher than the vapor deposition in literature. This characteristic favors the formation of dense and uniform film (Fig. 2a).
We also found that the as-deposited film forms Sb-S(e) chemical bonds, which enables the formation of high quality Sb2(S,Se)3 film at low annealing temperature. Since both the hydrothermal deposition and post-annealing temperature are not high, they are 150 oC and 350 oC, respectively, the interfacial diffusion can be alleviated between absorber layer Sb2(S,Se)3 and electron transporting layer CdS (Fig. 2b). The crystal orientation is also tunable to certain extent for favorable charge transport towards the electrodes (Fig. 2c), more selenourea in the reaction system leads to high degree of [hk1] orientation so that the charge transport is favored.
The hydrothermal deposition provides an effective approach for the synthesis of Sb2(S,Se)3 films, fine tuning the reactive deposition parameter with suitable interfacial engineering seems promising for further improving the efficiency.
Please read us at https://www.nature.com/articles/s41560-020-0652-3
Follow the Topic
-
Nature Energy

Publishing monthly, this journal is dedicated to exploring all aspects of this on-going discussion, from the generation and storage of energy, to its distribution and management, the needs and demands of the different actors, and the impacts that energy technologies and policies have on societies.
Related Collections
With Collections, you can get published faster and increase your visibility.
Microgrids and Distributed Energy Systems
Publishing Model: Hybrid
Deadline: Mar 31, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in