Assessing comparative Non-Human Primate models for SARS-CoV-2 infection and Coronavirus Disease 2019 (COVID-19)
Published in Microbiology

The novel coronavirus SARS-CoV-2 emerged a year ago and has since led to a major pandemic, COVID-19. Since the Southwest National Primate Research Center at Texas Biomedical Research Institute is home to multiple NHP species and breeding colonies, and we specialize in infectious diseases research using NHPs, we embarked on the new challenge of performing comparative NHP model development for SARS-CoV-2 infection. The overarching goal was to rapidly establish an appropriate NHP model and utilize it to test candidate therapeutics and vaccines for COVID-19.
With the combined efforts from exceptional researchers with diverse background and varied skillsets, preliminary studies reported here1 were rapidly completed. These studies clearly established that rhesus macaques demonstrated clinical features of infection over 3 days which resolved over two weeks. Baboons demonstrated a similar viral kinetics but with more severe pathological observations while marmosets were less prone to infection1. Both macaques and baboons demonstrated early signs of SARS-CoV-2 infection which resolved over a period of 14-17 days (Figure 1) with persistence of viral antigens and RNA in tissues but a marked absence of replicative virus. The age didn’t exhibit a drastic variation in terms of disease development and pathological manifestations however older macaques demonstrated a distinct immune-profile with a marked lower levels of SARS-CoV-2-Spike specific plasma IgG concentrations1; lower Follicular T helper cells frequencies (unpublished); downregulation of VEGF signaling; lower upregulation of Type I IFN and Notch signaling in endpoint lung tissues2. When compared with macaques, baboons exhibited more pronounced age-related effects, developed more severe inflammatory lesions and exhibited prolonged viral shedding.
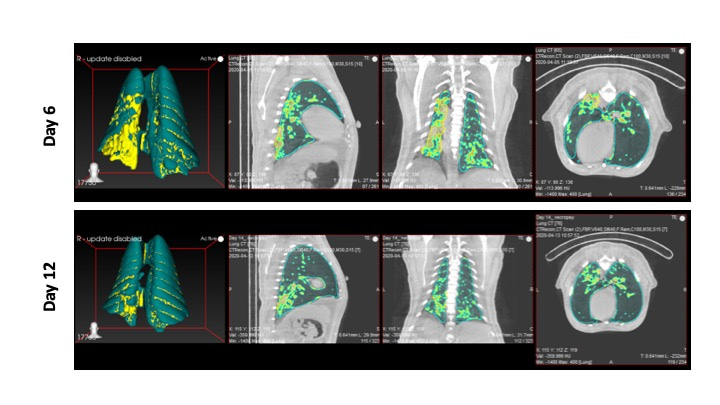
Figure 1. Three-dimensional (3D) reconstruction of the region of interest (ROI) volume representing the location of the lesions in a SARS-CoV-2 infected Rhesus macaque after 6 and 12 days post infection.
Image Credit: Shashank Ganatra & Xavier Alvarez, Southwest National Primate Research Center.
With a wider repertoire of immunological reagents available for rhesus macaques, this species appears more suitable for vaccine and therapeutic evaluations, while baboons appears to be seminal for evaluating co-morbidities associated with COVID-19. Over the course of preceding month this models has already been successfully used to evaluate a leading candidate vaccine (Pfizer/BioNTec B2)3 and therapeutic intervention (Regeneron antibody cocktail)4 for COVID-19. This model is now being utilized to assess the efficacy of numerous other vaccine candidates. It is also important to identify strategies which will permit greater pathogenesis in these models, allowing us to assess immunotherapeutic evaluations.
Read more on our findings here.
References:
1. Singh, D. K. et al. Responses to acute infection with SARS-CoV-2 in the lungs of rhesus macaques, baboons and marmosets. Nature Microbiology, doi:10.1038/s41564-020-00841-4 (2020).
2. Rosa, B. A. et al. IFN signaling and neutrophil degranulation transcriptional signatures are induced during SARS-CoV-2 infection. bioRxiv, doi:10.1101/2020.08.06.239798 (2020).
3. Vogel, A. B. et al. A prefusion SARS-CoV-2 spike RNA vaccine is highly immunogenic and prevents lung infection in non-human primates. bioRxiv, 2020.2009.2008.280818, doi:10.1101/2020.09.08.280818 (2020).
4. Baum, A. et al. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science 370, 1110-1115, doi:10.1126/science.abe2402 (2020).
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in