Automating Inhibition Zone Detection with YOLOv8n and LabVIEW Integration
Published in Bioengineering & Biotechnology, Computational Sciences, and General & Internal Medicine
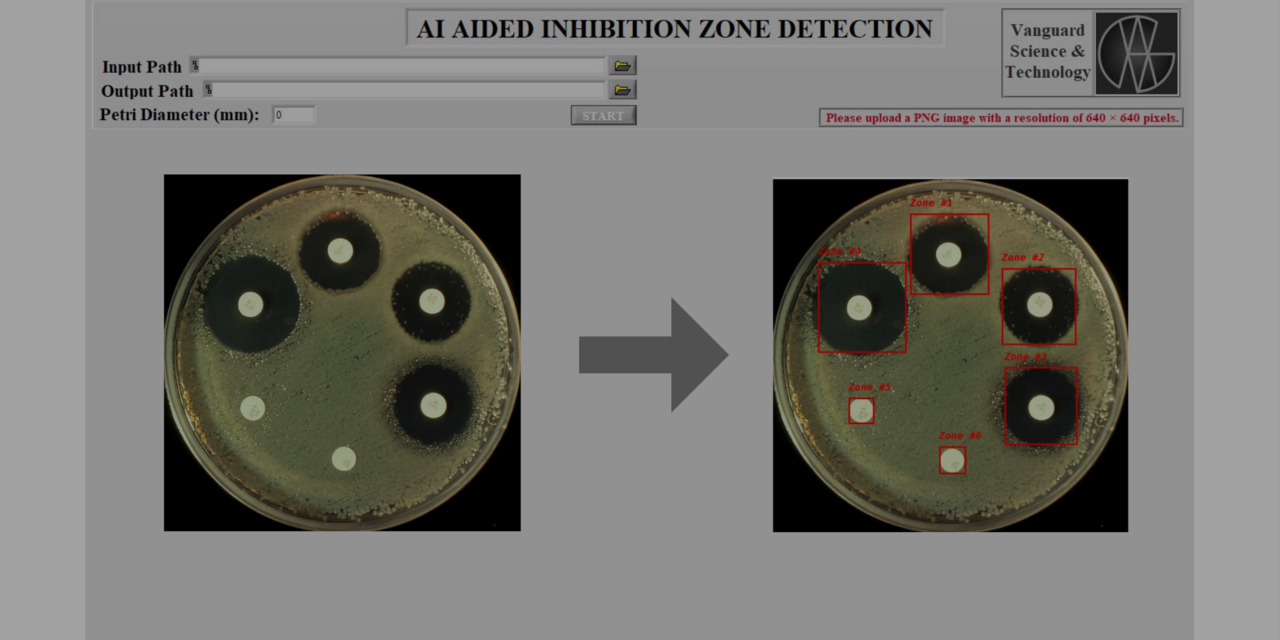
Antibiotic resistance remains one of the greatest challenges in modern medicine. A cornerstone in monitoring bacterial susceptibility is the disk diffusion assay, where the inhibitory effect of antibiotics is assessed by measuring the inhibition zone around an antibiotic disk placed on a Petri dish. Despite its importance, the evaluation of inhibition zones is still often performed manually. Such measurements are labor-intensive, subject to human error, and limited by inter-observer variability.
Recent advances in artificial intelligence (AI) and computer vision provide promising solutions to overcome these limitations. In this work, I developed an automated detection and measurement system for inhibition zones by integrating the YOLOv8n object detection model with LabVIEW, enabling real-time analysis and creating a standalone application that can be deployed in microbiology laboratories.
From Manual Assessment to Automated Detection
Traditional assessment of inhibition zones involves visually inspecting Petri dishes, identifying the zone of bacterial growth inhibition, and measuring its diameter with a ruler or caliper. This method, although widely used, is inherently subjective and time-consuming.
By contrast, an automated approach ensures:
-
Accuracy: Consistent detection of inhibition zones, independent of the observer.
-
Speed: Instant analysis of Petri dish images.
-
Reproducibility: Standardized measurements that can be validated and compared across laboratories.
This transformation from manual to automated workflows reflects a broader shift in experimental microbiology, where AI and automation are increasingly integrated into routine practice.
Step 1: Training YOLOv8n for Inhibition Zone Detection
The pipeline begins with training the YOLOv8n (You Only Look Once, version 8 – nano) model. A dataset of Petri dish images was annotated to mark both the dish boundaries and the inhibition zones.
The annotated dataset was then used to fine-tune YOLOv8n. After training, the best-performing model was exported as best.pt, a weight file optimized for inhibition zone detection. The model demonstrated the capability to robustly identify both the circular Petri dish and the surrounding inhibition halo.
Step 2: Python Implementation for Measurement
A Python script was developed to utilize the trained weights. The workflow proceeds as follows:
-
Input Image: A captured photograph of a Petri dish.
-
Detection: YOLOv8n identifies the Petri dish boundary and the inhibition zone.
-
Measurement: The diameter of the inhibition zone is calculated relative to the known Petri dish size, ensuring scale accuracy.
-
Visualization: Results are rendered and displayed as annotated images.
This Python-based detection pipeline provided the analytical foundation for the next phase: integration with LabVIEW.
Step 3: LabVIEW Integration for User-Friendly Operation
While Python offers flexibility and rapid prototyping, laboratory settings often require more structured and user-friendly environments. To bridge this gap, the Python script was embedded into a LabVIEW interface.
The integration enabled the following features:
-
Automated execution of Python code within LabVIEW.
-
Real-time visualization of detection results on a LabVIEW dashboard.
-
User interactivity, allowing researchers with minimal coding background to operate the system seamlessly.
This design made the workflow accessible beyond computer scientists — directly supporting microbiologists and clinical researchers.
Step 4: Building an Executable Application
To further enhance usability, the entire workflow was compiled into an executable (.exe) file. This transformation eliminated the need for Python installations or external dependencies, allowing the program to run independently on laboratory computers. By doing so, the project effectively evolved from a research prototype into a standalone application, ready for deployment in real-world microbiology laboratories. For a short visual demonstration of the workflow, please see the accompanying video on our Vanguard Science and Technology Instagram page.
Broader Impact and Future Perspectives
The automated inhibition zone detection system represents more than a technical innovation; it demonstrates how AI can be translated into practical laboratory tools.
Key benefits include:
-
Faster workflows, reducing manual workload for microbiologists.
-
Improved accuracy, minimizing measurement inconsistencies.
-
Scalability, enabling integration into high-throughput antimicrobial testing pipelines.
Looking forward, this approach can be extended to other experimental assays where precise measurement is required, such as colony counting, biofilm quantification, or cell culture monitoring. Integrating AI into laboratory workflows opens the door to smarter, reproducible, and automated science, bridging the gap between computational innovation and bench-side application.
Conclusion
By combining YOLOv8 object detection, Python automation, and LabVIEW integration, this project delivers a robust and accessible solution for inhibition zone analysis. The transition from manual measurement to automated detection highlights the transformative role of AI in microbiology, offering new possibilities for faster, more accurate, and reproducible laboratory research. This work exemplifies how cutting-edge AI techniques can be embedded into experimental practice, contributing to more efficient workflows and improved scientific outcomes.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in