B-Bridge Regulated Asymmetric Dual-Atomic Catalysts for Synergistically Enhanced Styrene Mineralization and CO2 Reduction
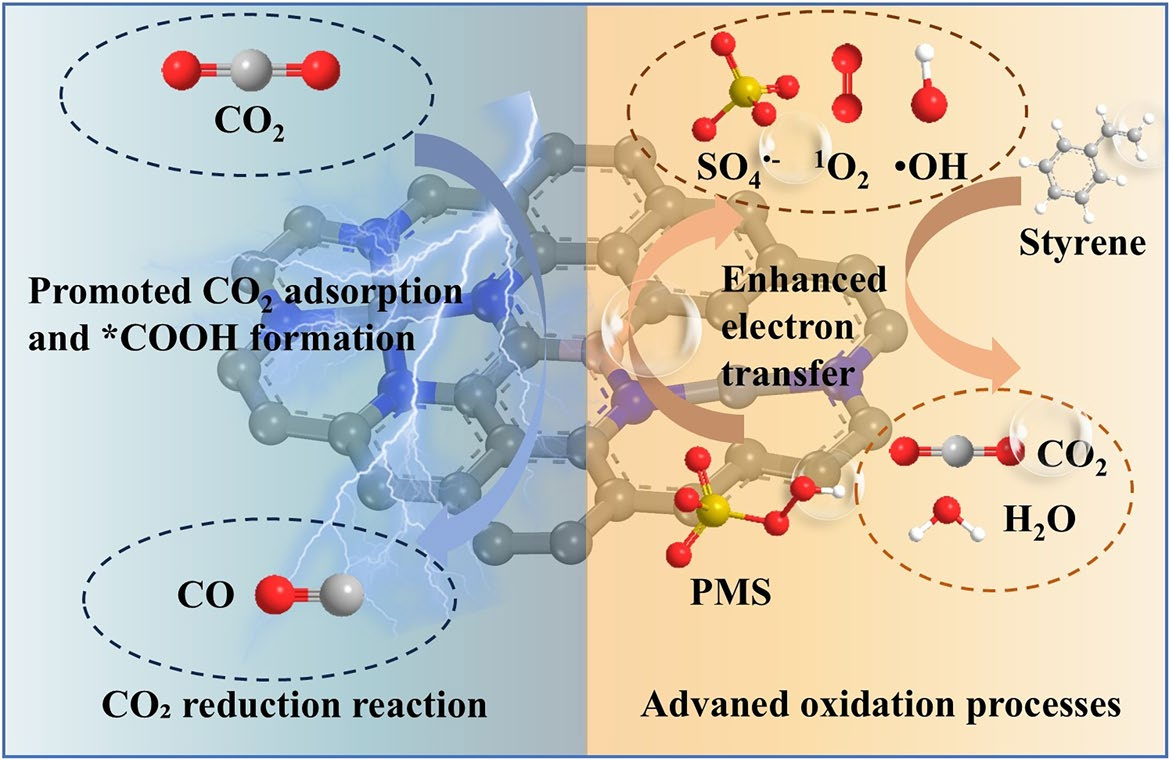
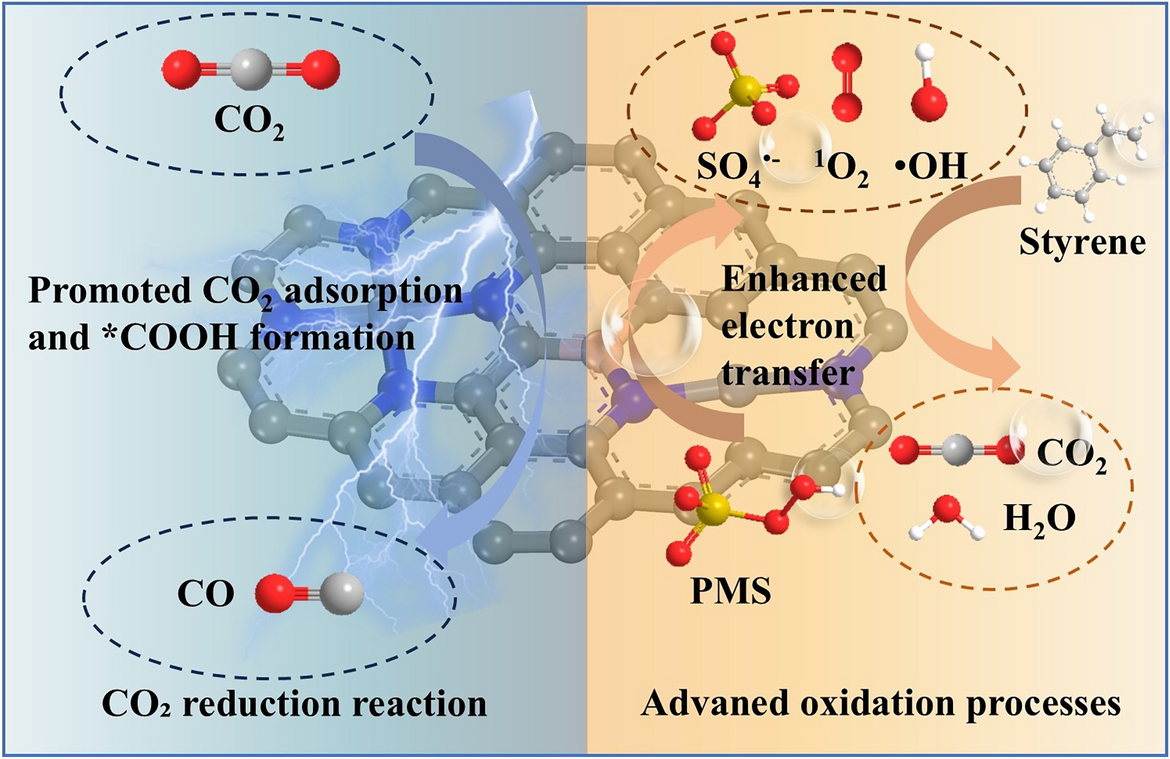
A research team led by Professor Chuncai Kong from Xi’an Jiaotong University has designed boron-bridge regulated asymmetric dual-atomic catalysts (NiFe-BNC) that deliver exceptional bifunctional activity for volatile organic compound (VOC) degradation and electrochemical CO2 reduction. Published in Nano-Micro Letters, this work demonstrates a sustainable platform for converting waste gases into valuable resources through synergistic catalysis.
Why This Catalyst Matters
- Unprecedented Performance: NiFe-BNC achieves 99% continuous styrene degradation in 2 hours with stable mineralization above 60%. Simultaneously, it enables CO2 electroreduction with a Faradaic efficiency of 98% at industrial-scale current densities of 1000 mA cm-2.
- Boron Electron Bridge: Boron doping induces carbon defects, enhances PMS adsorption, and optimizes electronic coupling between Ni and Fe sites, enabling accelerated electron transfer.
- Dual Functionality: The same catalyst platform simultaneously addresses environmental remediation (VOC oxidation) and energy conversion (CO2-to-CO).
Design Strategy
The NiFe-BNC catalyst is derived from chitosan, a biomass-based substrate rich in amino and hydroxyl groups that stabilize dispersed metal atoms. Secondary calcination with boric acid introduces a “boron electron bridge” which modulates local coordination environments and electronic density. This design yields:
- Defect Engineering: Increased pyridinic nitrogen and carbon defects promote PMS activation.
- Optimized Interfaces: Strong Fe–Ni–B coupling tunes intermediate adsorption energies for both VOC oxidation and CO2.
- Hierarchical Porosity: A stable porous structure enables rapid diffusion of reactants and products.
Mechanistic Insights
- VOC Degradation: PMS is activated on NiFe-BNC to generate reactive oxygen specie, which drive styrene mineralization. In situ Raman and electrochemical analyses confirm enhanced charge transfer compared with undoped catalysts.
- CO2 Reduction: Density functional theory (DFT) calculations reveal that Fe–Ni dual sites with B modulation lower the energy barrier for *COOH formation (from 2.18 to 0.65 eV) and weaken CO adsorption, thereby improving CO selectivity and suppressing hydrogen evolution.
- Electronic Modulation: Projected density of states and charge density mapping confirm that boron introduces asymmetric charge redistribution, enhancing electron transfer kinetics and stabilizing dual-atom coordination.
Performance Highlights
- VOC Removal: 99% styrene degradation with >60% CO2 mineralization in flowing systems.
- CO2 Electroreduction: 98% CO Faradaic efficiency at 1000 mA cm-2 with long-term stability.
- Industrial Viability: Outperforms conventional single-atom and dual-atom catalysts, providing a cost-effective route for coupled environmental and energy applications.
Future Outlook
This study establishes a blueprint for multifunctional catalyst design via electron-bridge regulation. By integrating biomass-derived supports, dual-metal active centers, and heteroatom doping, the NiFe-BNC system demonstrates scalable potential for both pollutant remediation and carbon recycling, advancing the vision of closed-loop carbon utilization.
Stay tuned for further developments from Professor Chuncai Kong and the collaborators as they continue to explore innovative materials for a greener tomorrow.
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in