Behind the paper: Elucidating reaction dynamics in lithium–sulfur batteries via operando x-ray diffraction of hollow carbon sphere hosts
Published in Chemistry, Materials, and Mechanical Engineering

Department of Physics | Department of Chemical Sciences | Bernal Institute | Faculty of Science and Engineering | University of Limerick
Background.
Rechargeable batteries are ubiquitous in modern life. From user electronics, lifesaving medical devices, and electric transportation, batteries are crucial in supporting the technology-driven modern world. Lithium-ion batteries (Li-ion) have dominated the rechargeable energy storage space since the late 90s; however, their energy storage capacity is limited by the underlying chemistry. The development of alternative battery systems is essential to facilitate rapidly growing demands.
Lithium–sulfur batteries (Li–S) are at the forefront of “beyond Li-ion” technologies, offering roughly 5× the energy storage capacity. This makes them ideal for long-distance electric vehicles, including electric aviation, as well as the large-scale storage of renewable energy for grid integration. However, there remain critical limitations that prevent the widespread adoption of Li–S batteries.
In this paper, we explored a green-chemistry approach for electrode materials production and investigated their impact on Li–S battery performance using operando X-ray diffraction. This study was a result of a collaboration between the University of Limerick and the University of the Basque Country (UPV/EHU).

The story behind our paper.
The seed for this research was planted when two ideas collided: (i) How can we synthesise electrode materials that maintain the cost and environmental benefits that the sulfur chemistry brings? (ii) What impact do these materials have on the underlying reaction dynamics of an operating Li–S battery?
The incorporation of a sulfur host material into Li–S cathodes is a long-established strategy for improving battery performance. By melting sulfur into a carbon structure, you create a composite material with a significantly increased electrical conductivity and structural integrity, allowing for a better utilisation of sulfur and improved long-term stability. Unfortunately, the current strategies for synthesising carbon host materials are infeasible for commercial applications, due to significant complexity, cost, and environmental concerns associated with them. To tackle this, we developed a low-cost, scalable, and renewable sugar-based alternative.
To answer the second question, we developed a suite of advanced operando characterisation techniques, including X-ray diffraction and Raman spectroscopy, within the McNulty Group, the Department of Physics, and the Bernal Institute. These techniques allow us to investigate the microscopic structure of the battery while it is charging and discharging, leading to an in-depth understanding of the internal mechanisms. This is particularly important in the case of sulfur batteries, as the conversion reaction leads to a cascade of intermediate species, culminating in a complete structural change between charged and discharged states.

A sustainable methodology for hollow carbon spheres.
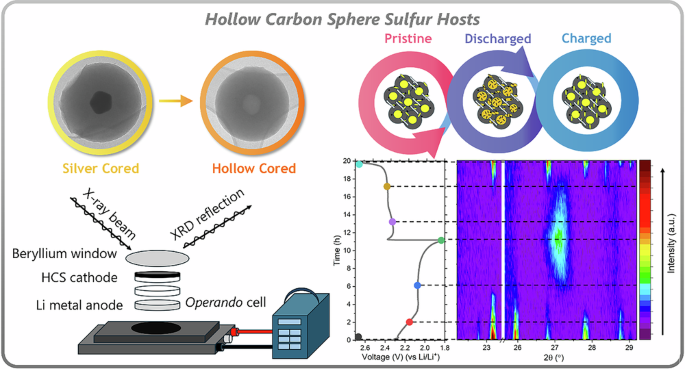
Sugars (e.g. glucose, sucrose, fructose) offer an inexpensive, renewable, and safe source of carbon that can be converted into novel functional materials. This puts them in a unique position to replace petroleum-derived products, a vital step in the transition to a renewables-based economy. We wanted to develop a simple method for converting glucose into hollow carbon spheres and found that hydrothermal treatment was the most promising pathway. The hydrothermal method involves heating water above 100°C inside a sealed container (Teflon-lined autoclave), leading to extreme pressures that can drive reactions not typically achievable under standard conditions. For the case of glucose, this can drive the formation of aromatised carbon spheres. By incorporating silver nitrate into the reaction solution, silver nanoparticles formed first and acted as seeds for carbon sphere deposition, leading to the production of a silver-cored carbon sphere structure. This sparked an idea: If we can etch the silver nanoparticle with nitric acid, we can produce hollow-carbon spheres and a silver nitrate waste solution. Silver nitrate can easily be recovered from the waste using previously established methodology, and reused for future synthesis batches, a key principle of circular manufacturing. After some trials, tribulations, and iterations, we developed a robust, repeatable process and had prepared enough material for Li–S battery testing.
Peering into a battery: Operando characterisation.
For me, this is the best part of the research! Operando analysis allows us to track the material changes that occur during cycling, giving us direct access to the underlying mechanisms at play. In turn, these insights can be used to make informed decisions on future material design. X-ray diffraction was our technique of choice because it gives detailed crystallographic information. This is particularly informative for Li–S batteries as the mechanism contains the formation and dissolution of multiple crystals, including orthorhombic sulfur (α-S8), monoclinic sulfur (β-S8), and cubic lithium sulfide (Li2S). In the video attached, you can see how these crystals (peaks in the diffractogram) disappear and reappear during a full cycle of the Li–S battery.
I still remember when we first looked at the results! A feeling of genuine discovery! From previous studies in the literature, it’s common to see the formation of β-S8 “needles” after charging; however, we saw the formation of a reoriented α-S8 species. This is a denser and more thermodynamically stable species, which is likely contributing to the improved cyclability of our electrodes. We were also curious to see if we could detect evidence of an amorphous Li2S2 material, which is currently believed to be a key contributor to the lower-than-theoretical discharge capacity in Li–S batteries. This is challenging because its non-crystalline nature makes it indetectable by X-ray diffraction. Instead, we looked for its influence on surrounding materials and found that it significantly limits Li2S crystallite growth during discharge.
Final thoughts.
Li–S batteries have a key role to play in the future of sustainable energy, enabling long-distance electric vehicles and the grid-integrated storage of renewable energy. Developing scalable, green synthesis pathways for electrode materials is essential for realising the full potential of Li–S batteries while maintaining the inherent low-cost and environmental advantages. In this study we utilised glucose, as an alternative to petroleum-derived materials, to synthesise hollow carbon sphere electrode materials.
Given the vast complexity of the sulfur reaction mechanism, a deep understanding of the underlying dynamics is vital to overcome limitations and provide insights for future material and cell designs. In this work, we used operando X-ray diffraction to delve into the precipitation reactions that underscore Li–S charge storage. Our observations raise new knowledge on the influence of crystal allotropes and orientation on electrochemical performance.
For more details, the full journal article is available open access in Communications Materials: https://doi.org/10.1038/s43246-025-00883-3.
Deaglán Bowman is a PhD student on the ALTERNATE Project, in the Department of Chemical Sciences, University of Limerick.
Dr. Erlantz Lizundia is Associate Professor at the School of Engineering in Bilbao, University of the Basque Country (UPV/EHU).
Dr. David McNulty is Associate Professor of Energy Materials and Devices in the Department of Physics, University of Limerick.
Follow the Topic
-
Communications Materials

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of materials science.
Related Collections
With Collections, you can get published faster and increase your visibility.
Advanced characterizations of high-entropy materials
Publishing Model: Open Access
Deadline: Mar 31, 2026
Multifunctional hydrogels
Publishing Model: Open Access
Deadline: Feb 28, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in