Behind the Paper: How PRICKLE3 Found Its Place in WNT/PCP Signalling
Published in Biomedical Research
Starting with cells and a simple question
The project started in our lab at Masaryk University in Brno with a basic but persistent question: what is the specific role of PRICKLE3 in the WNT/planar cell polarity (PCP) pathway?
While PRICKLE1 and PRICKLE2 have been studied extensively, PRICKLE3 was often considered less important or poorly defined. We were not fully convinced by this view, and early experiments in human cell lines already suggested that PRICKLE3 behaves differently from the other family members.
At this early stage, much of the experimental work was driven by Katarzyna (Kasia) Radaszkiewicz, whose careful and systematic approach helped shape the direction of the project.
A turning point: proteomics
A major shift came when we decided to use proximity-dependent biotinylation (miniTurboID) combined with mass spectrometry. This allowed us to move beyond candidate interactions and map PRICKLE protein environments in an unbiased way.
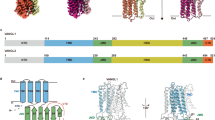
With strong support from the CEITEC proteomics core facility and close collaboration with bioinformatic experts, we generated comparative interactomes of PRICKLE1, PRICKLE2, and PRICKLE3. The result was striking: PRICKLE3 displayed a clearly distinct interaction network, strongly enriched in core non-canonical WNT/PCP components, including VANGL1 and VANGL2.
This finding changed the scope of the project. What started as a cell biology study became a mechanistic investigation of PCP receptor regulation.
From interactions to mechanism
We then focused on understanding what PRICKLE3 actually does. Using inducible cell lines, biochemical assays, and imaging, we found that PRICKLE3 stabilizes VANGL proteins at the plasma membrane.
Mechanistically, PRICKLE3 reduces CK1ε-mediated phosphorylation of VANGL and protects it from RNF43-dependent ubiquitination and degradation. Importantly, this function was specific to PRICKLE3 and was not observed for PRICKLE1, highlighting functional specialization within the PRICKLE family.
Many of these experiments were technically demanding and required careful optimization and repetition. Progress was steady, but not always fast — a familiar situation in long-term projects.
Extending the work in vivo
To test whether these mechanisms are relevant beyond cell culture, we expanded the study to vertebrate embryos. Xenopus allowed us to validate VANGL stabilization in vivo, while zebrafish embryos enabled direct visualization of PCP-related membrane dynamics during development.
At this stage, international collaboration became essential. Colleagues in Singapore contributed expertise in zebrafish biology and advanced imaging, adding an important in vivo dimension to the work and strengthening the overall conclusions.
A long but rewarding process
This project developed over several years, during which the team evolved and new challenges appeared. Bringing all experimental, proteomic, and in vivo data together into a coherent story required persistence, coordination, and a strong collaborative spirit.
Why it matters
Beyond identifying a new role for PRICKLE3, this work reveals a previously unrecognized regulatory layer controlling the stability of PCP receptor complexes. By linking PRICKLE3 to CK1ε and RNF43, we provide new insight into how non-canonical WNT signalling is fine-tuned during development and disease.
We also hope that the datasets and molecular tools generated in this study will be useful for the wider community.
Final thoughts
Behind this paper is a story of curiosity, teamwork, and long-term commitment. It also shows that proteins considered “minor” can play central roles when studied with the right tools and sufficient patience.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
A link to the paper is here: PRICKLE3 protects VANGL proteins from CK1-mediated phosphorylation and RNF43-mediated degradation | Communications Biology