Beyond Particles: Discovering Gas-Phase Formation of Organic Sulfate
Published in Earth & Environment

An Unexpected Discovery in Eastern China
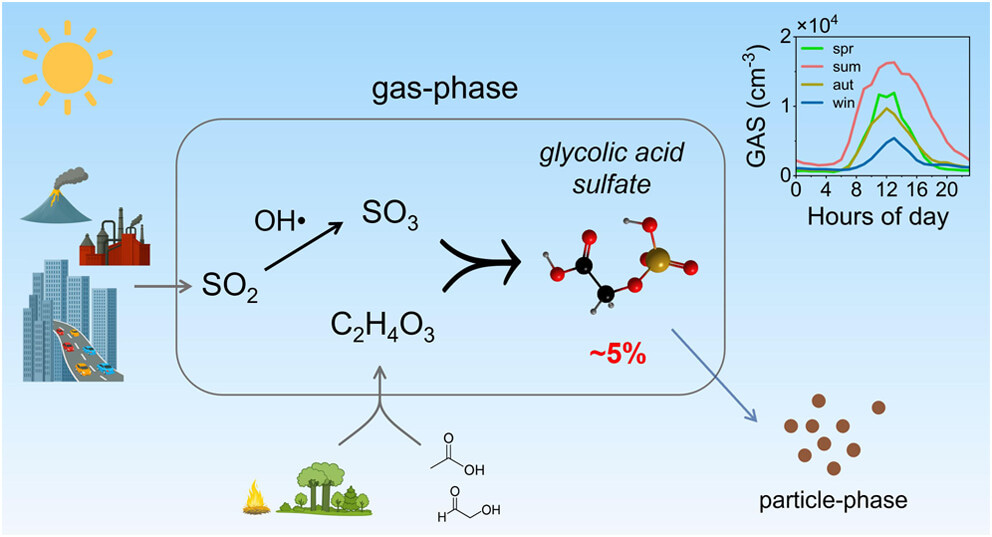
The research team from Nanjing University made their discovery while conducting comprehensive atmospheric measurements across multiple sites in eastern China using high-resolution mass spectrometry. What caught their attention was the presence of glycolic acid sulfate (GAS) in the gas phase, with concentrations that followed clear diurnal patterns, peaking during daylight hours with maximum values reaching 4.6 × 10⁴ molecules cm⁻³ during summer.
This observation was puzzling. According to thermodynamic principles, GAS should partition almost entirely into the particle phase. The compound's measured maximum desorption temperature of approximately 128.9°C places it firmly in the ELVOC category—molecules that should stick to particles rather than exist freely in the gas phase.
Yet the measured gas-phase concentrations exceeded equilibrium predictions by several orders of magnitude, indicating that simple evaporation from particles could not explain the observations.
Identifying the Formation Mechanism
Through systematic analysis, the researchers uncovered crucial clues about the formation process. Gas-phase GAS concentrations showed significant positive correlations with sulfur trioxide (SO₃), glycolic acid, and—most importantly—the product of these two species' concentrations. This relationship strongly suggested a bimolecular gas-phase reaction between SO₃ and glycolic acid.
Using observationally constrained data, the team determined a reaction rate constant of approximately 2.2 × 10⁻¹⁰ cm³ s⁻¹ for the SO₃ + glycolic acid reaction. This near-collision-limit rate indicates that the reaction proceeds with high efficiency when the two molecules encounter each other.
The discovery represents the first field-based evidence for this reaction pathway, which had been previously suggested by theoretical quantum chemical calculations but never demonstrated in ambient environments.
Broader Implications
The gas-phase formation pathway contributes approximately 15.6% to the total particle-phase GAS burden. This finding extends beyond a single compound and raises fundamental questions about our understanding of organic sulfate formation in the atmosphere.
If GAS can form via gas-phase reactions despite its extremely low volatility, other organic sulfates might follow similar pathways. This discovery could potentially reshape our understanding of secondary organic aerosol formation and new particle formation processes.
The efficient gas-phase formation of organic sulfates has particular relevance for environments rich in both sulfur compounds and organic precursors. Urban areas, volcanic plumes, forest environments, and wildfire emissions—where anthropogenic or natural SO₂ sources coincide with organic compounds—may serve as hotspots for such reactions.
This work exemplifies how careful field observations can reveal gaps in our theoretical understanding of atmospheric processes. As analytical techniques continue to advance and measurement campaigns expand globally, we can expect further discoveries that deepen our understanding of atmospheric chemistry. The atmosphere, it appears, still holds surprises even for compounds we believed we understood completely.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in