α-Branched amines by catalytic 1,1-addition of C–H bonds and aminating agents to terminal alkenes
Published in Chemistry
Multi-component reactions, which combine three or more coupling partners, are powerful transformations in organic synthesis because they enable the rapid generation of complex structures from simple precursors. We have recently begun to explore a new three-component coupling approach for the synthesis of organic compounds by C–H bond activation and sequential addition across two different coupling partners. We have previously reported sequential C–H bond additions to enones and aldehydes (Angew. Chem. Int. Ed. 2016, 55, 12650-12654), alkynes and halogenating agents (Angew. Chem. Int. Ed. 2017, 56, 9976–9980), and more recently to dienes and aldehydes (Nat. Catal. 2018, 1, 673–679).
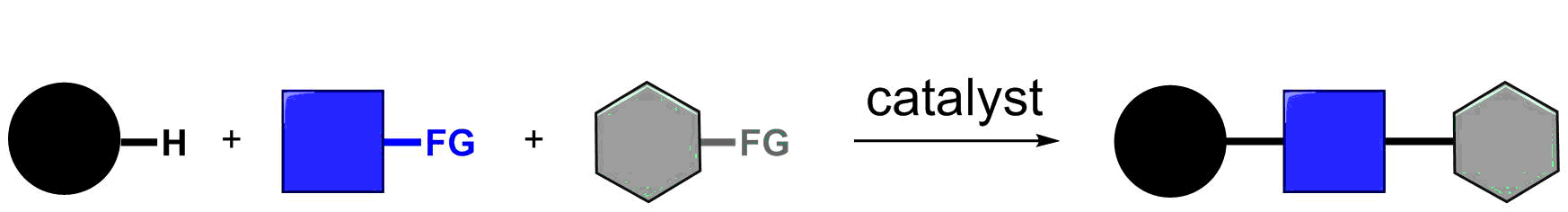
Transition metal-catalyzed three-component C–H bond addition
In carrying out the current work we had two primary goals. First, we sought to use alkenes as one of the coupling partners due to the very large number and variety of alkenes that are commercially available. However, we recognized that alkenes were likely to be challenging partners because they would generate unstabilized alkyl organometallic intermediates. Second, because amines are incorporated into a majority of drug structures, we were also very interested in preparing amine products by employing aminating agents as a second coupling partner (Chem. Rev. 2017, 117, 9247–9301). As we began to investigate sequential C–H bond additions to alkenes and aminating agents, we encountered two unexpected findings. We were surprised that Cp*Rh(III) catalysts were much more effective than Cp*Co(III) catalysts because Cp*Co(III) catalysts had always worked best for our previously reported three-component C–H bond additions. The reaction also proceeded by an unexpected 1,1-addition to the alkene rather than much more common 1,2-addition. This reaction outcome can be understood based upon the mechanistic studies described in our article and as supported by a rigorous study on rhodacycle equilibration reported by Bill Jones and his coworkers (Organometallics 2010, 29, 4593–4605). We look forward to further developing this three-component approach for the rapid synthesis of amines from readily available inputs. We are also exploring many other types of three-component coupling reactions that might also proceed by 1,1-alkene addition to provide access to different types of reaction products.
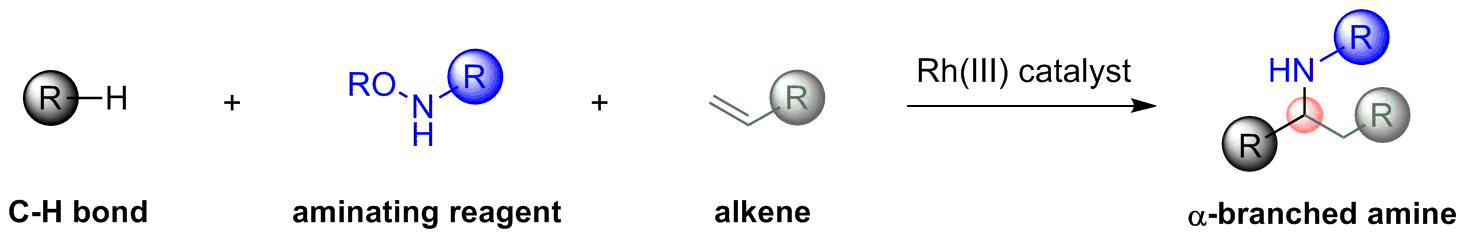
Rh(III)-catalyzed three-component 1,1-addition to alkenes to give α-branched amines
For more details please take a look at our paper “α-Branched amines by catalytic 1,1-addition of C–H bonds and aminating agents to terminal alkenes” in Nature Catalysis.
Follow the Topic
-
Nature Catalysis

This journal brings together researchers from across all chemistry and related fields, publishing work on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, incorporating both fundamental and applied studies.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in