Bridging the gap: immunobridging large-scale efficacy data of a tetravalent dengue vaccine (TAK-003) from children and teenagers to adults
Published in Microbiology, General & Internal Medicine, and Pharmacy & Pharmacology
Our article published in NPJ Vaccines1 describes our approach to addressing one of the most important challenges in vaccine development, ie, gauging vaccine efficacy in situations where large-scale clinical trials are not feasible. We encourage you to read the article to gain a deeper understanding of our research. Here, we discuss challenges for development of a dengue vaccine candidate in the context of the results of our study.
Dengue and challenges in vaccine development
Half of the world’s population is estimated to be at risk for dengue, a mosquito-borne viral disease that is a rising global health concern, with cases of dengue and impacted areas predicted to increase over time as a result of climate change and urbanization.2-4 People become infected with dengue when they are bitten by a mosquito carrying any one of four types of dengue virus, namely dengue virus 1 through 4.5 The multiplicity of dengue virus types contributes to the complexity of dengue immunology and poses challenges for vaccine development.6
Traditionally, most cases of dengue occur during childhood, but dengue infection is rising in adults,7 in part as a result of successful mosquito-control and disease-monitoring programs.8 While many cases of dengue infection are asymptomatic or result in mild symptoms (fever, headache, skin rash and/or pain), some people experience severe disease that can lead to bleeding and death.2,3 Importantly, adults are more likely to have comorbidities that may increase the risk for development of severe dengue.9,10 Furthermore, people can experience a repeated episode of symptomatic dengue infection when exposed to a different dengue virus type, which may result in more severe dengue in certain circumstances.6 TAK-003 is a live-attenuated, tetravalent dengue vaccine candidate developed to protect against all four types of dengue virus.11 Data from the ongoing DEN-301 phase 3 trial (TIDES; NCT02747927) of TAK-003 have shown protection against virologically confirmed dengue and prevention of consequent hospitalization and a favorable benefit/risk profile in children and teenagers from areas where dengue is common (ie, endemic areas) regardless of whether they had a previous dengue infection at initial vaccination.11-14
One of the challenges in developing a dengue vaccine is conducting efficacy trials, which require large numbers of participants, in all populations for whom it is intended. In endemic areas, most people are exposed to dengue during childhood or adolescence limiting the number of adults who have not been exposed (ie, seronegative) available to participate in efficacy trials.15,16 In areas where dengue is not common (ie, non-endemic areas), exposure to dengue is not expected, thus making assessment of efficacy of a candidate vaccine in these areas not feasible. Dengue immunology is complex making it important to understand the potential efficacy of a vaccine in seronegative people across the lifespan, especially because adults represent an important vulnerable population for dengue infection.
Addressing the challenges in dengue vaccine development
Immunobridging is an established methodology for providing information on efficacy in populations when clinical trials are impractical or not feasible, and it has been used successfully in the development of multiple approved vaccines.17-19 This approach is commonly used to predict the efficacy of candidate vaccines and circumvents the need for large phase 3 clinical trials, allowing time- and cost-efficient development of vaccines to fill critical unmet needs.20 Immunobridging takes vaccine efficacy data established under one set of conditions and extrapolates it to infer efficacy under a different set of conditions.21 Across the TAK-003 clinical development program, clinical trials in endemic areas mostly enrolled children and adolescents, whereas, clinical trials in non-endemic areas exclusively enrolled adults. We addressed the challenge of lack of vaccine efficacy data in adults from endemic areas by conducting an immunobridging analysis between the immunogenicity data from the DEN-301 trial, which was conducted in children and teenagers aged 4–16 years who lived areas in endemic areas (ie, Latin America and Asia), and the DEN-304 (NCT03423173) phase 3 trial of TAK-003 in adults aged 18-60 years from the United States (US) where dengue is non-endemic. This analysis only included participants who did not have evidence of a previous dengue infection before they enrolled in the trial so that the two populations were more closely matched, except for age.
Another challenge in the development of vaccines is between-assay variability and the importance of a standardized method for measuring neutralizing antibody levels when comparing the immune response to vaccination between studies and different groups of people. The microneutralization (MNT) assay, which assesses levels of dengue neutralizing antibodies, is the accepted, reproducible method of assessing immune response to dengue vaccine.22 The immunogenicity of TAK-003 was evaluated in both the DEN-301 and DEN-304 trials using the MNT assay to determine the levels of neutralizing antibodies in blood serum. Our immunobridging analysis was based on neutralizing antibody levels measured using the dengue MNT assay, which overcomes the important limitation of between-assay variability when comparing results across trials.
Findings from our immunobridging analysis
To predict the efficacy of TAK-003 in seronegative adults, we compared the level of dengue-neutralizing antibodies (ie, geometric mean titers [GMTs]) and the proportion of people who were dengue-neutralizing antibody-positive above a specific threshold (ie, seropositivity rate) between children/adolescents and adults in the two studies for each of the four types of dengue virus at months 4 and 9 (ie, one and six months after the second vaccination). The neutralizing antibody titer was considered similar if the upper bound of the 95% confidence interval (CI) for the geometric mean ratio (GMR) between the two age groups was < 2.0. Results from the analysis showed similar neutralizing antibody levels between the age groups for dengue virus types 1, 2, and 4 at Month 4, and for all four dengue virus types by Month 9 (Figure 1). At Month 4, the adult age group had lower neutralizing antibody titers than children/adolescents just missing the 95% CI threshold for non-inferiority, but by Month 9 the neutralizing antibody levels were similar between the age groups.
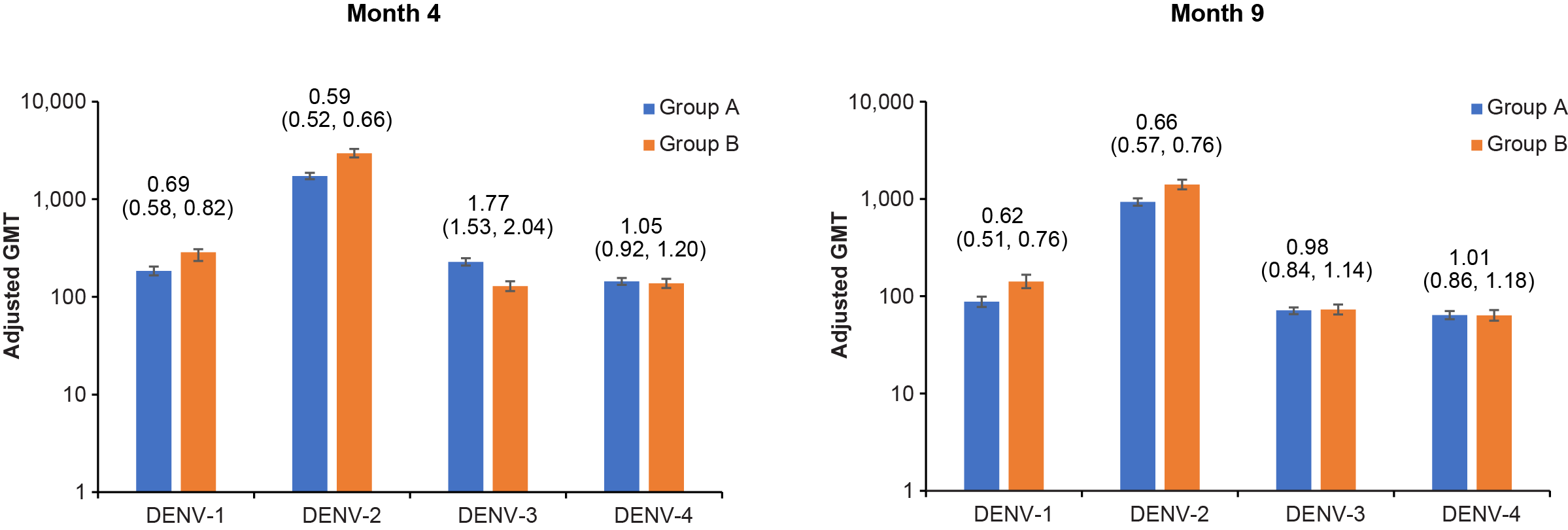
Figure 1. Neutralizing antibody titers against each dengue virus subtype by age group. The blue bars (Group A) are data from participants aged 4–16 years from the DEN-301 trial and the orange bars (Group B) from participants aged 18–60 years from the DEN-304 trial. The height of the bars represents the adjusted GMT of dengue neutralizing antibody for each dengue virus type assessed using the microneutralization assay. The values above the bars are the adjusted GMR (95% CI) for each dengue virus type. If the upper bound of the 95% CI for a GMR was < 2.0 then the immunogenicity between the age groups was considered similar. Abbreviations: CI, confidence interval; DENV, dengue virus; GMR, geometric mean ratio; GMT, geometric mean titer.
The distributions of neutralizing antibody levels among participants were largely similar between age groups across dengue virus types, although a slightly higher proportion of adults had higher titers against dengue virus 1 and 2 compared with children and teenagers and a slightly higher proportion of children and teenagers than adults had higher titers for dengue virus type 3. The distributions of neutralizing antibody levels were comparable for dengue virus type 4 in both age groups. There were no clear differences or trends in neutralizing antibody levels with increasing age (Figure 2). Regardless of age group, seropositivity rates were high at months 4 and 9 (range: 92%–100%), and more than 95% of individuals had neutralizing antibodies against all 4 types of dengue virus one month after vaccination.
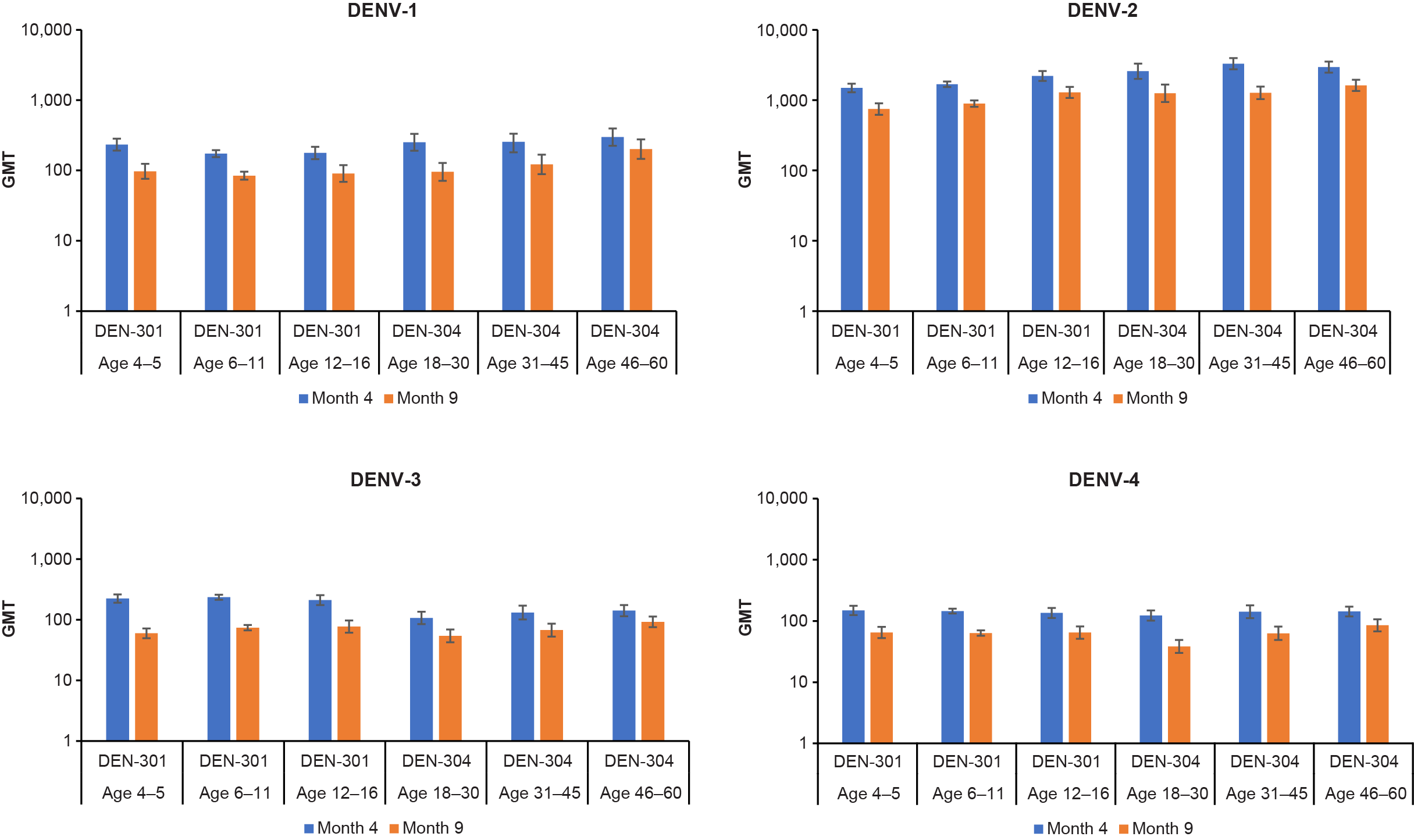
Figure 2. Neutralizing antibody titers against each dengue virus subtype by age subgroup in seronegative participants. The blue bars are data from Month 4 and the orange bars from Month 9. The height of the bars represents the GMT of dengue neutralizing antibodies for each dengue virus type assessed using the microneutralization assay; the error bars are the 95% confidence intervals. Abbreviations: DENV, dengue virus; GMT, geometric mean titer.
The threshold for the level of neutralizing antibody or other immunologic response that protects against future dengue virus infection (ie, a correlate of protection) has not yet been defined. Establishing the correlate of protection for dengue has been challenging because infection induces immune responses specific to the type of dengue virus that caused the infection, but also cross-reactive immune response to the other dengue virus types. Given this, we conducted additional exploratory analyses to characterize a range of immune responses consistent with antiviral and flavivirus immunity that may contribute to protection from infection. This included type-specific and cross-reactive neutralizing antibody levels, binding antibody levels, anti-dengue complement-fixing antibody levels, tetravalent antibody binding strength, and antibody response to non-structural protein 1, a conserved viral protein. These analyses were conducted in a subset of 48 participants from each trial and showed comparable immune responses between the two age groups and support the findings from the main analyses.
Taken together, the findings from our immunobridging analysis show similar immune responses between age groups and support extrapolating the efficacy profile of TAK-003 demonstrated in children and teenagers to the adult population in protecting against dengue infection.
Key take aways
- Under circumstances where placebo-controlled trials are not feasible or are impractical, immunobridging is an established alternative to evaluate vaccine efficacy in subpopulations of interest.
- The low number of seronegative adults in regions where dengue is common and low rates of dengue infection in areas where dengue is not common necessitates immunobridging the efficacy TAK-003 from that established in seronegative children and adolescents to seronegative adults and avoids any complications introduced by prior dengue infections.
- Immunobridging between data from a clinical trial of TAK-003 in seronegative children and adolescents and a clinical trial of TAK-003 in seronegative adults showed similar immune responses to TAK-003 in these populations.
- These findings support extrapolating the efficacy of TAK-003 demonstrated in children and teenagers to the adult population.
Acknowledgements
We thank all our coauthors for their contributions to the development of the npj Vaccines article, the clinical trial participants and their parents or guardians, and the principal investigators and staff at the clinical trial sites. Under direction of the contributors, Alison Gagnon, PhD of Excel Medical Affairs (part of Envision Pharma Group) provided medical writing support and editorial assistance for this blog. Takeda Pharmaceuticals provided funding to Excel Medical Affairs for support in writing and editing this blog.
References
- LeFevre, I., et al. Bridging the immunogenicity of a tetravalent dengue vaccine (TAK-003) from children and adolescents to adults. NPJ Vaccines 8, 75 (2023).
- European Centre for Disease Prevention and Control. Dengue factsheet. Vol. 2023 (2021).
- World Health Organization. Dengue and severe dengue. Vol. 2023 (2023).
- Messina, J.P., et al. The current and future global distribution and population at risk of dengue. Nat Microbiol 4, 1508-1515 (2019).
- Halstead, S.B. Pathogenesis of dengue: challenges to molecular biology. Science 239, 476-481 (1988).
- Elong Ngono, A. & Shresta, S. Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development. Front Immunol 10, 1316 (2019).
- Tantawichien, T. Dengue fever and dengue haemorrhagic fever in adolescents and adults. Paediatr Int Child Health 32 Suppl 1, 22-27 (2012).
- Ang, L.W., Cutter, J., James, L. & Goh, K.T. Seroepidemiology of dengue virus infection in the adult population in tropical Singapore. Epidemiol Infect 143, 1585-1593 (2015).
- Pang, J., Hsu, J.P., Yeo, T.W., Leo, Y.S. & Lye, D.C. Diabetes, cardiac disorders and asthma as risk factors for severe organ involvement among adult dengue patients: A matched case-control study. Sci Rep 7, 39872 (2017).
- Toledo, J., et al. Relevance of Non-communicable Comorbidities for the Development of the Severe Forms of Dengue: A Systematic Literature Review. PLoS Negl Trop Dis 10, e0004284 (2016).
- Biswal, S., et al. Efficacy of a Tetravalent Dengue Vaccine in Healthy Children and Adolescents. N Engl J Med 381, 2009-2019 (2019).
- Biswal, S., et al. Efficacy of a tetravalent dengue vaccine in healthy children aged 4-16 years: a randomised, placebo-controlled, phase 3 trial. Lancet 395, 1423-1433 (2020).
- Lopez-Medina, E., et al. Efficacy of a Dengue Vaccine Candidate (TAK-003) in Healthy Children and Adolescents 2 Years after Vaccination. J Infect Dis 225, 1521-1532 (2022).
- Rivera, L., et al. Three-year Efficacy and Safety of Takeda's Dengue Vaccine Candidate (TAK-003). Clin Infect Dis 75, 107-117 (2022).
- Watanaveeradej, V., et al. Transplacentally transferred maternal-infant antibodies to dengue virus. Am J Trop Med Hyg 69, 123-128 (2003).
- Pengsaa, K., Limkittikul, K., Yoksan, S., Wisetsing, P. & Sabchareon, A. Dengue antibody in Thai children from maternally transferred antibody to acquired infection. Pediatr Infect Dis J 30, 897-900 (2011).
- Essink, B., et al. Pivotal Phase 3 Randomized Clinical Trial of the Safety, Tolerability, and Immunogenicity of 20-Valent Pneumococcal Conjugate Vaccine in Adults 18 Years and Older. Clin Infect Dis (2021).
- Lowy, D., Herrero, R. & Hildesheim, A. Chapter 1. Summary of IARC/NCI Expert Meeting on Primary End-points for Prophylactic HPV Vaccine Trials. in IARC HPV Working Group. Primary End-points for Prophylactic HPV Vaccine Trials (International Agency for Research on Cancer, Lyon, France, 2014).
- US Food & Drug Administration: Vaccines and Related Biological Products Advisory Committee Meeting. FDA Briefing Document: Licensure and Emergency Use Authorization of Vaccines to Prevent COVID-19 for Use in Pediatric Populations. Vol. 2022 (2021).
- Khoury, D.S., et al. Correlates of Protection, Thresholds of Protection, and Immunobridging among Persons with SARS-CoV-2 Infection. Emerg Infect Dis 29, 381-388 (2023).
- Fritzell, B. Bridging studies. Dev Biol Stand 95, 181-188 (1998).
- Carpp, L.N., et al. Microneutralization assay titer correlates analysis in two phase 3 trials of the CYD-TDV tetravalent dengue vaccine in Asia and Latin America. PLoS One 15, e0234236 (2020).
Follow the Topic
-
npj Vaccines

A multidisciplinary journal that is dedicated to publishing the finest and high-quality research and development on human and veterinary vaccines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Lipid nanoparticle (LNP)-adjuvanted vaccines
Publishing Model: Open Access
Deadline: Feb 19, 2026
Therapeutic HPV vaccines
Publishing Model: Open Access
Deadline: Jun 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in