Cancer cells steal energy from the immune system by intercellular nanotubes
Published in Bioengineering & Biotechnology

Even though immunotherapies have emerged as the standard cancer treatment in the clinic, about half of the patients still do not respond to them1. Immune nonresponsiveness mainly occurs due to the lack of functional immune cells that could be boosted by immunotherapy. While this could happen due to the intrinsic absence/scarcity of functional immune cells in the tumor microenvironment, cancer cells also actively harness immune suppressive mechanisms to deplete pre-existing immune cells. Restoration of a functional immune system in the tumor microenvironment can significantly amplify the effectiveness of immunotherapy.
We rationalized that observing the interaction between cancer cells and immune cells at the nanoscale could provide new insight into the cancer immune suppressive mechanisms. During the experiments, we particularly paid attention to preserving cellular physical structures to explore the possible unknown intercellular communication mechanisms that would occur in 3D tissue context in vivo, beyond classical paracrine signaling mechanisms or direct junction-based juxtacrine mechanisms. Every step involved during the culturing cells and analyzing their interactions was carried out extremely gently to conserve cellular structures as much as possible. After a careful effort, excitingly, we discovered the presence of long nanotubes between cancer cells and immune cells, which had a completely distinguished feature from a general cytoskeletal structure of an individual cell (Fig 1)2. These nanotubes had a thickness of ~50 nm-2 µm and length of ~3-100 µm, which can be up to several cell diameters, and were physically bridging the surfaces of cancer cells and immune cells.
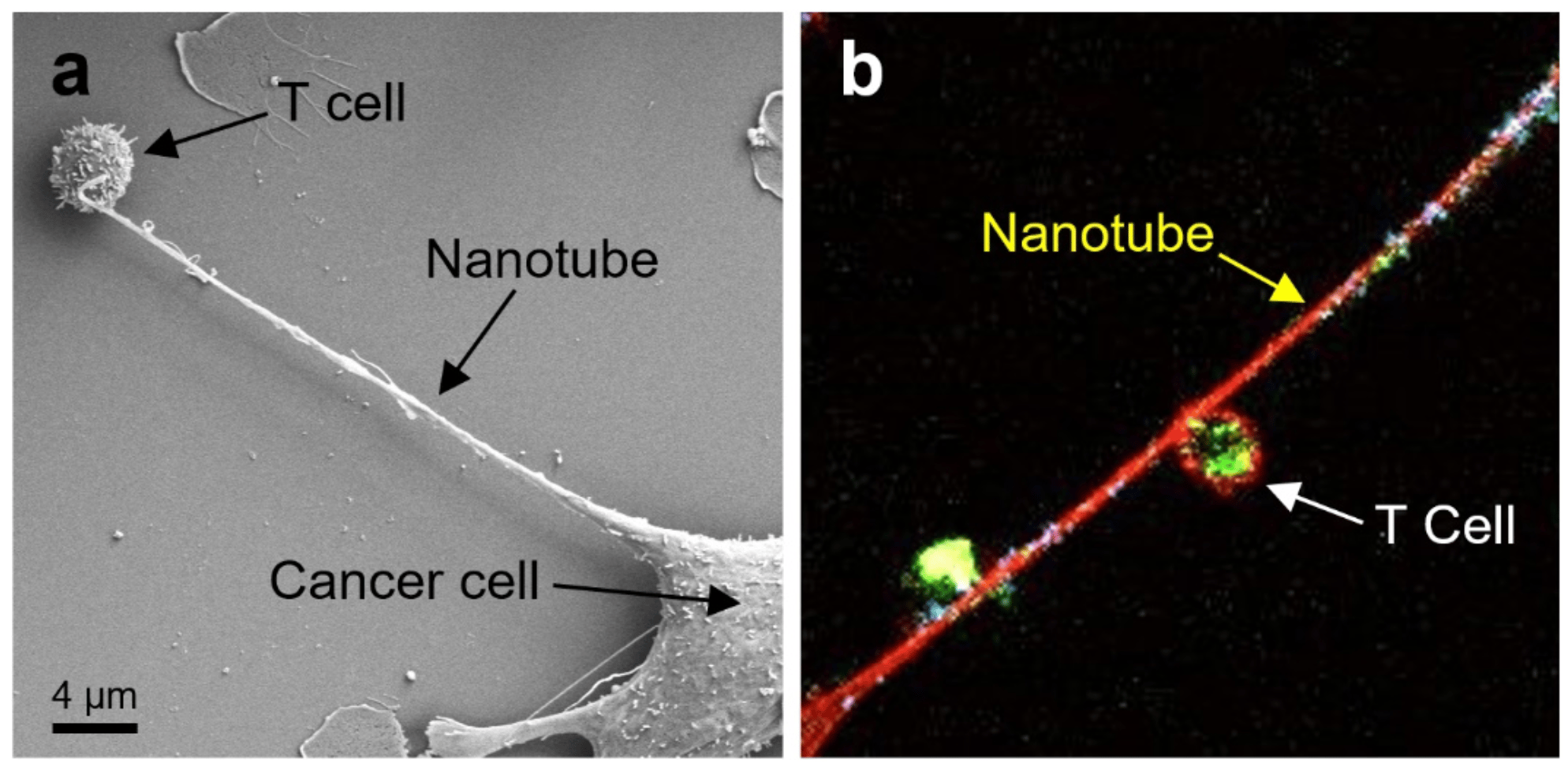
Figure 1. a) Field emission scanning electron microscopy (FESEM) image showing intercellular nanotube formation between a cancer cell and an immune cell. b) Confocal microscopy image visualizing mitochondria traveling from an immune cell to a cancer cell through the physical nanotube. Mitochondria, DNA in the mitochondria, and actin were stained green, blue, and red, respectively. Figure from our work2.
What would be the purpose of these extraordinarily long nanotubes between cancer cells and immune cells? Construction of such noticeably long-range intercellular highways would require significant biological efforts from the cells. After conducting several screenings to identify the component of these nanotubes, we found that these nanotubes were composed of actin cytoskeletal elements and, strikingly, acting as an intercellular pathway for the transportation of mitochondria, which are the energy sources of cells. Mitochondria transport via intercellular nanotube was predominantly one-way direction from immune cells to cancer cells, indicating that cancer cells were stealing energy sources from immune cells. Mitochondria are essential for the metabolism in cells, affecting their growth and activities. Mitochondria-gained cancer cells are reported to accelerate cancer progression and develop resistance to chemotherapy. Indeed, our study showed that cancer cells that hijacked mitochondria from immune cells exhibit augmented metabolic rates with increased growth rates. On the other hand, immune cells that lost mitochondria by cancer cells become metabolically depleted and show decreased growth rate. When we prevented the formation of nanotubes between cancer cells and immune cells by using a pharmacological inhibitor, the number of activated T cells was significantly increased and the effectiveness of the anti-tumor immunotherapy was augmented, confirming that the nanotube-mediated energy hijacking of cancer cells from immune cells could act as one of the major cancer immune suppression mechanisms.
Our study has opened a new perspective in cancer immune suppression by presenting an additional cellular interaction mechanism between cancer cells and immune cells mediated by long-distance physical nanotubes. In fact, nanotube-like structures have been implicated in intercellular communication among various types of cells, which could be beneficial or detrimental for cells depending on the conditions3. Viral/bacterial pathogens and prions can hijack nanotubes for intercellular disease transmission4-6. On the other hand, healthy stem cells use intercellular nanotubes to transfer mitochondria to damaged stromal cells to restore their health7. Cancer cells also share mitochondria among themselves to rescue apoptotic cancer cells and increase the survival rate of tumors in a multicellular context8. Cancer cells further utilize nanotubes to manipulate stromal cells to support tumor growth and invasiveness. Previously, our group reported that cancer cells dynamically regulate endothelium by forming nanobridges and transferring mRNAs to convert healthy endothelium into pathological endothelium that acts as metastatic foci9. Pharmacological perturbation of these nanotubes not only significantly decreased metastatic foci but also transformed the phenotype of cancer cells into a less invasive agglomerated form from highly invasive elongated feature9,10.
In our current study, we showed that cancer cells were hijacking mitochondria from immune cells using physical nanotubes, causing immune suppression2. While it was clear that the mitochondria transport between cancer cells and immune cells was facilitated by the formation of nanotubes, we could not conclude whether these mitochondria were going through the internal portion of the nanotubes or along the surface of the nanotubes (or even both). In fact, the intercellular nanobridges that we observed were the aggregation of nanotubes, while some of them were too thin to allow the penetration of mitochondria within the tubes. Our FESEM images further showed the presence of numerous organelles on the surface of the nanotubes that had a similar shape to mitochondria (Fig 2). Clarification of the location of mitochondria during nanotube-mediated trafficking and their locomotion principles remain open questions.
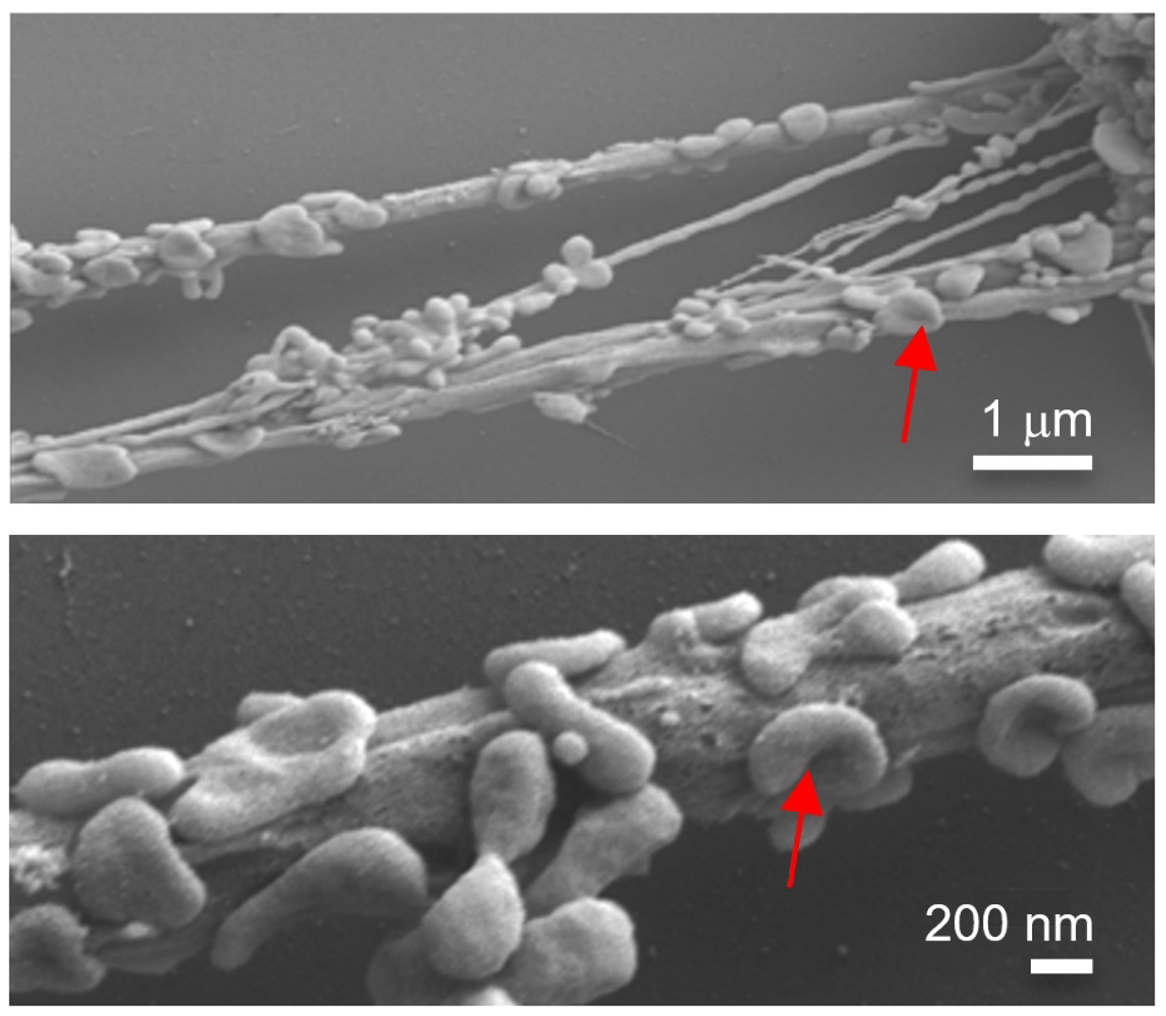
Figure 2. Field emission scanning electron microscopy (FESEM) images showing the cellular organelle-like structures (red arrow) on the nanotubes formed between breast cancer cells and immune cells.
Our discovery also raised advanced questions: Do cancer cells hijack energy from any types of stroma cells for their own growth, or their energy stealing is particularly targeting immune cells to suppress the immune system? In addition, is that the cancer cells form this long tentacle to actively search for energy sources and cleverly kill its predators without exposing its main body to danger, or are these nanobridges/tubes established when the two cells were physically contacting and gradually elongate as these two cells migrate into separate directions? Would an aggregate of cancer cells develop a spider network of nanotubes to trap and counterattack individual immune cells? Advanced in vitro human tissue modeling systems can facilitate addressing these questions by directly visualizing cellular communications in the tissue context at the nanoscale in real time. Biological insights obtained from such systems can lead to next-generation therapies. These advanced in vitro human tissue modeling systems allows the evaluation of innovative therapies and elucidation of therapeutic mechanisms in physiologically/pathologically relevant stromal context, which cannot be easily achieved in classical 2D cell culture system11. We envision that complete comprehension of cancer immune suppression mechanisms will enable the realization of the full potential of existing immunotherapies in the clinic to overcome cancers.
The original article can be found in,
Saha, T., Dash, C., Jayabalan, R. et al. Intercellular nanotubes mediate mitochondrial trafficking between cancer and immune cells. Nat. Nanotechnol. (2021). https://doi.org/10.1038/s41565-021-01000-4
References:
1 Dammeijer, F., Lau, S. P., van Eijck, C. H., van der Burg, S. H. & Aerts, J. G. Rationally combining immunotherapies to improve efficacy of immune checkpoint blockade in solid tumors. Cytokine & growth factor reviews 36, 5-15 (2017).
2 Saha, T. et al. Intercellular nanotubes mediate mitochondrial trafficking between cancer and immune cells. Nature Nanotechnology, doi:10.1038/s41565-021-01000-4 (2021).
3 Rustom, A., Saffrich, R., Markovic, I., Walther, P. & Gerdes, H.-H. Nanotubular highways for intercellular organelle transport. Science 303, 1007-1010 (2004).
4 Gousset, K. et al. Prions hijack tunnelling nanotubes for intercellular spread. Nature cell biology 11, 328-336 (2009).
5 Sowinski, S. et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nature cell biology 10, 211-219 (2008).
6 Önfelt, B. et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. The Journal of Immunology 177, 8476-8483 (2006).
7 Ahmad, T. et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. The EMBO journal 33, 994-1010 (2014).
8 Wang, X. & Gerdes, H.-H. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death & Differentiation 22, 1181-1191 (2015).
9 Connor, Y. et al. Physical nanoscale conduit-mediated communication between tumour cells and the endothelium modulates endothelial phenotype. Nature communications 6, 1-14 (2015).
10 Dash, C., Saha, T., Sengupta, S. & Jang, H. L. Inhibition of Tunneling Nanotubes between Cancer Cell and the Endothelium Alters the Metastatic Phenotype. International Journal of Molecular Sciences 22, 6161 (2021).
11 Freag, M. S. et al. Human Nonalcoholic Steatohepatitis on a Chip. Hepatology Communications (2020).
Follow the Topic
-
Nature Nanotechnology

An interdisciplinary journal that publishes papers of the highest quality and significance in all areas of nanoscience and nanotechnology.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in