Cationic Adsorption‑Induced Microlevelling Effect: A Pathway to Dendrite‑Free Zinc Anodes
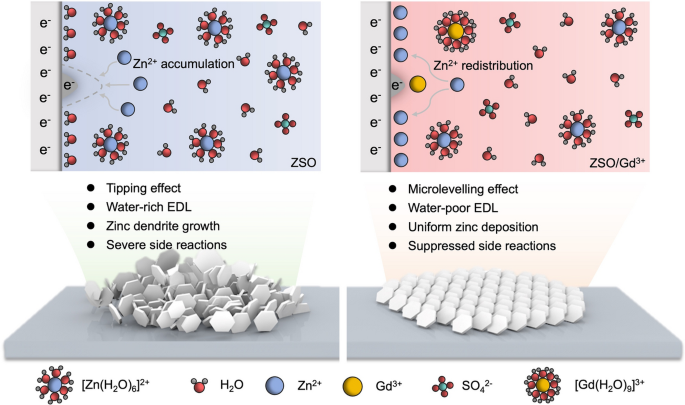
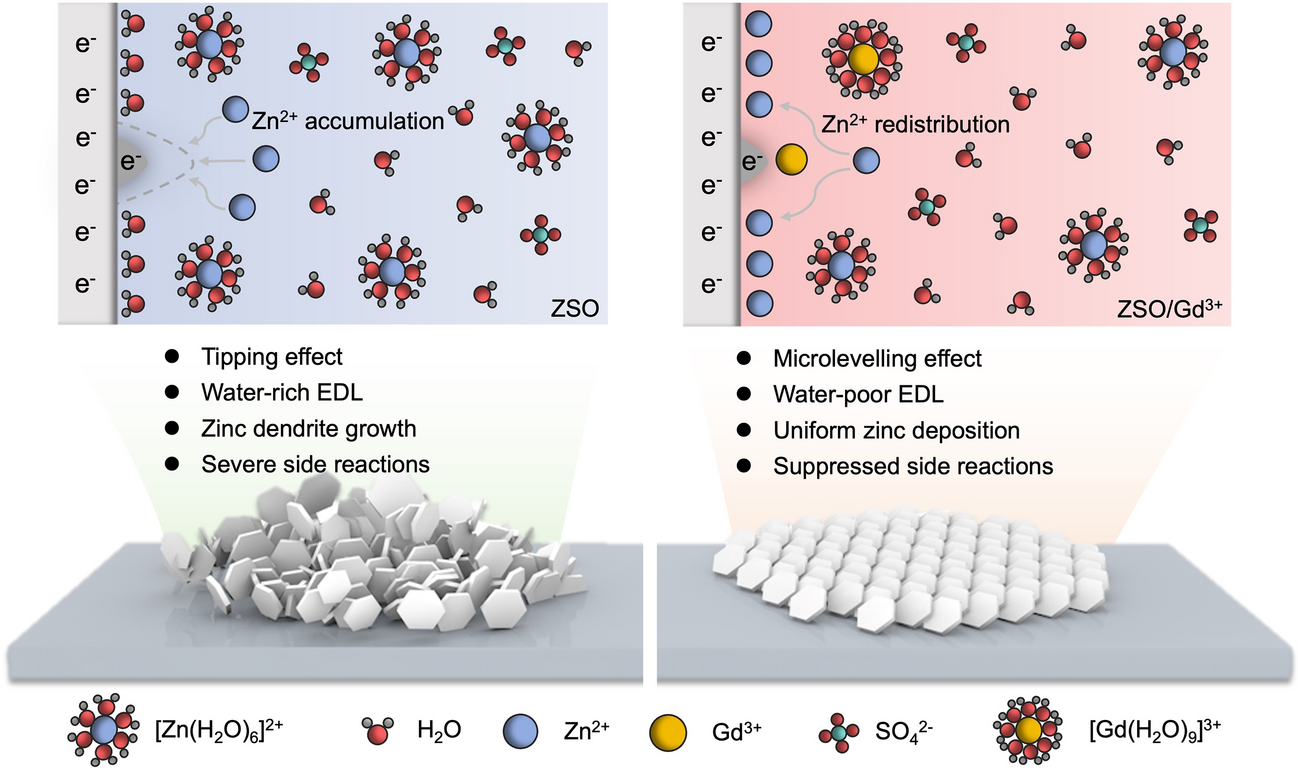
As aqueous zinc-ion batteries (AZIBs) edge closer to commercial scale-up, zinc-dendrite-induced shorting and corrosion remain the last big barriers. Now researchers from Central South University and CNPC Tubular Goods Research Institute, led by Prof. Jiang Zhou, Prof. Siyu Tian and Dr. Long Jiang, reveal that simply adding Gd3+ ions to a conventional ZnSO4 electrolyte triggers an in-situ “microlevelling” effect that eliminates dendrites for >2 000 h. The work delivers an immediately applicable electrolyte formula and a new physical picture for ultra-stable AZIBs.
Why Gd3+ Microlevelling Matters
• Dendrite-Free Deposition: Preferential adsorption of Gd3+ on Zn tips creates an electrostatic shield that redistributes Zn2+ flux, replacing the destructive “tipping” effect with a self-smoothing mechanism.
• Water-Poor Electric Double Layer: Gd3+ displaces interfacial H2O, suppresses HER and by-product (ZHS) formation, cutting corrosion current from 1.31 to 0.154 mA cm-2.
• Record Reversibility: Zn//Cu cells deliver 99.72 % average Coulombic efficiency over 1400 cycles; Zn//NH4V4O10 full cells retain 85.6 % capacity after 1000 cycles at 5 A g-1.
Innovative Design and Features
• Additive Chemistry: 0.05 M Gd2(SO4)3 in 3 M ZnSO4 (pH ≈ 1.6) — non-flammable, low-cost and compatible with existing cell assembly lines.
• Theoretical Insight: DFT shows Gd3+ adsorption energy on Zn(002) (−2.79 eV) far exceeds Zn2+ (−0.32 eV) and H2O (−0.14 eV), rationalizing the selective tip-blocking mechanism.
• Characterization Suite: In-situ optical microscopy, 3D confocal laser scanning and TOF-SIMS reveal surface roughness drop from 3.07 to 0.78 µm and uniform 3-D diffusion kinetics.
Applications and Future Outlook
• Symmetric Cells: Zn//Zn cells run >2100 h (1 mA cm-2, 1 mAh cm-2) and 1000 h (5 mA cm-2, 2 mAh cm-2) without shorting, outperforming La3+, Mg2+ and organic additive benchmarks.
• Full-Cell Validation: 2 × 2 cm2 pouch cells with NVO cathodes deliver 220 Wh kg-1 (cathode basis) and maintain 80 % capacity after 500 deep cycles at 2 A g-1.
• Challenges and Opportunities: Remaining work includes Gd supply-chain analysis, low-temperature performance and extension to high-voltage cathodes; the team is already testing Dy3+/Y3+ blends for further cost reduction.
This concise study provides an additive-level, factory-ready solution to the dendrite problem that has plagued AZIBs for a decade. It highlights how rare-earth cations—long used in zinc electroplating—can be repurposed to meet the stringent lifetime and safety demands of grid-scale storage. Stay tuned for more fast-charging, corrosion-free advances from Prof. Jiang Zhou and Prof. Siyu Tian at Central South University!
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in