Centenary of 1918 Influenza Pandemic | Snapshot: Nikki Shindo
Published in Microbiology

Name: Nahoko Shindo
Affiliation: Manager, Expert Networks
Health Emergencies Programme
World Health Organization
Geneva, Switzerland
Email: shindon@who.int
Website: www.who.int
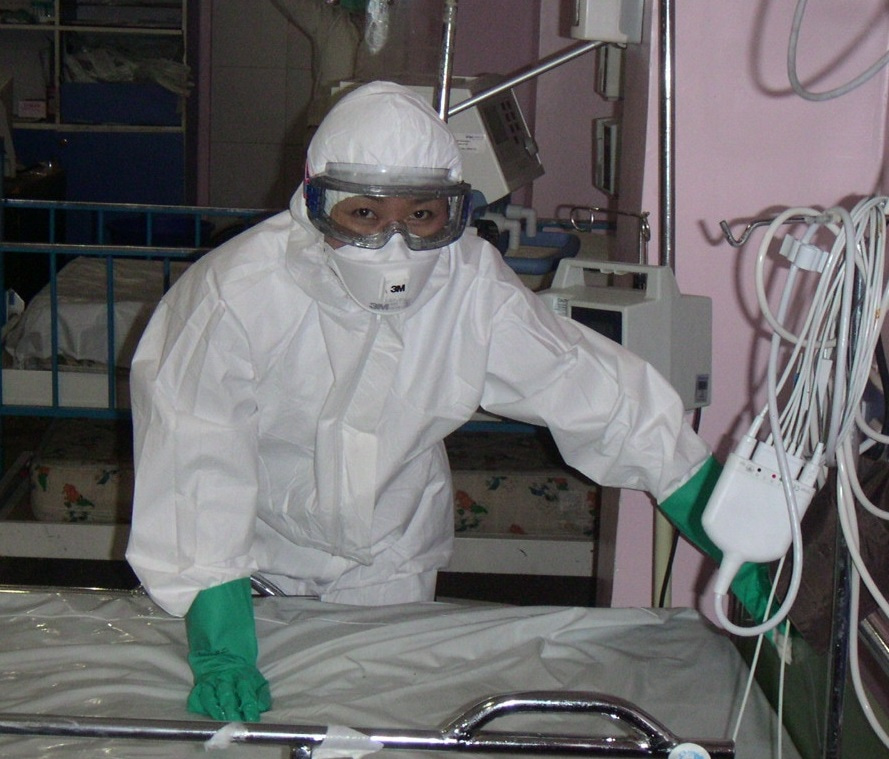
Could you tell me a bit about what your research/work entails?
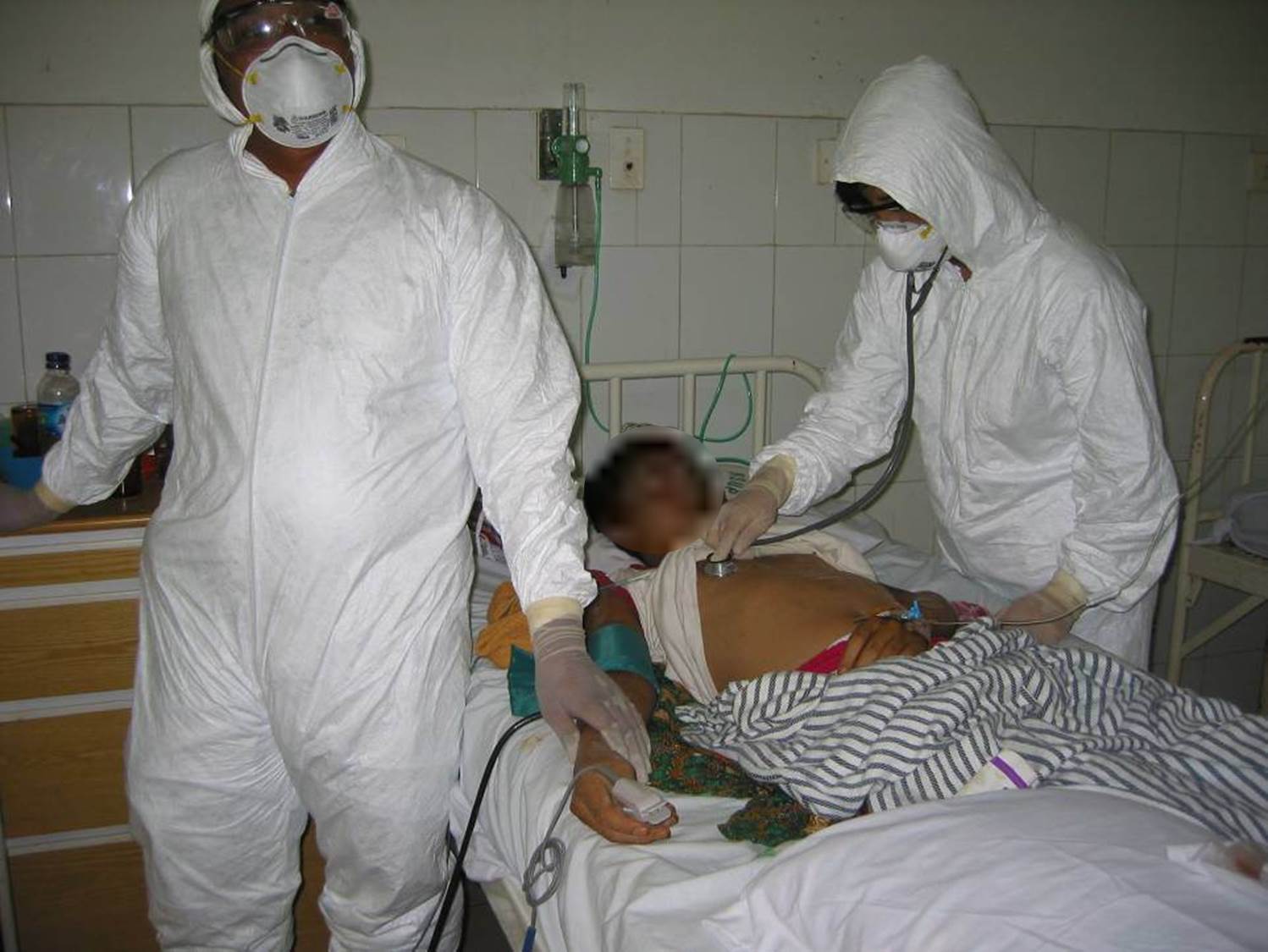
I am in charge of clinical aspects of influenza –in all forms: seasonal, zoonotic and pandemic– at the World Health Organization. I have been covering this since 2002. I witnessed the world marching of the H5N1 avian influenza in 2004-5, I investigated almost all outbreaks of human H5N1 infection, and was also directly involved in patient care in the hospitals (photos attached). I am in charge of developing WHO influenza clinical management guidance and WHO strategic global influenza antivirals stockpile deployment. At the beginning of the 2009 H1N1 influenza pandemic, we deployed 7 million doses of influenza antivirals to 72 eligible under-resourced countries within 14 days from the declaration of phase 4.

How did you become interested in influenza?
My involvement in influenza predates my WHO carrier. It began when I was a clinician at a teaching hospital in Japan, then my days at the National Institute of Infectious Diseases, Tokyo, Japan. There I worked to determine the burden of influenza disease and introduced the elderly vaccination program.
In what ways has the 1918 pandemic most influenced your research, and the wider virology and public health field?
What influenced me most was the elucidation of its pathophysiology and cause of severe illness and death. I was in charge of medical countermeasures, particularly influenza antivirals and severe respiratory distress patients management.
What do you see as the biggest accomplishments/breakthroughs in the field since the 1918 pandemic? Are there any papers that you feel are must reads for those that aren’t familiar with the field (and briefly, why)?
The biggest breakthroughs I think have come in the areas of vaccines, antivirals, and rapid diagnostics. A whole-society approach was proposed by WHO.
As references, I would suggest the 2009 WHO pandemic influenza preparedness guidance and the WHO Bulletin influenza themed issue editorial.
What do you see as the main challenges for research in your part of the field in the coming years?
The collection of well-planned clinical information. WHO and ISARIC (International Severe Acute Respiratory and Emerging Infection Consortium) developed a tool – standard data collection protocol (Clinical Characterization protocol, together with biological sampling strategies). Also the development of well-designed clinical trials for mono or combination therapies of influenza antivirals, as well as the use of monoclonal and polyclonal Abs as potential prevention and treatment strategies.
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in