Challenges in Second-Line Therapy for Advanced Pancreatic Cancer: The Search for Predictive Markers
Published in Cancer
Patients with advanced pancreatic (PDAC) cancer have a dismal prognosis. Of those failing 1st line chemotherapy, only about 50% proceed to 2nd line treatment. The decision to start 2nd line treatment is difficult for patients and providers because predictive markers for the efficacy of 2nd line treatment are rare.
The German AIO multicenter PREDICT-study evaluated predictive factors that are routinely collected during clinical care with the aim to support an informed and shared decision. Patients (n=151) previouly treated with gemcitabine/nab-paclitaxel were enrolled in a prospective single-arm trial and received biweekly nanoliposomal irinotecan/5-fluorouracil/folinic acid. Primary endpoint was TTF2, with secondary endpoints including overall survival.
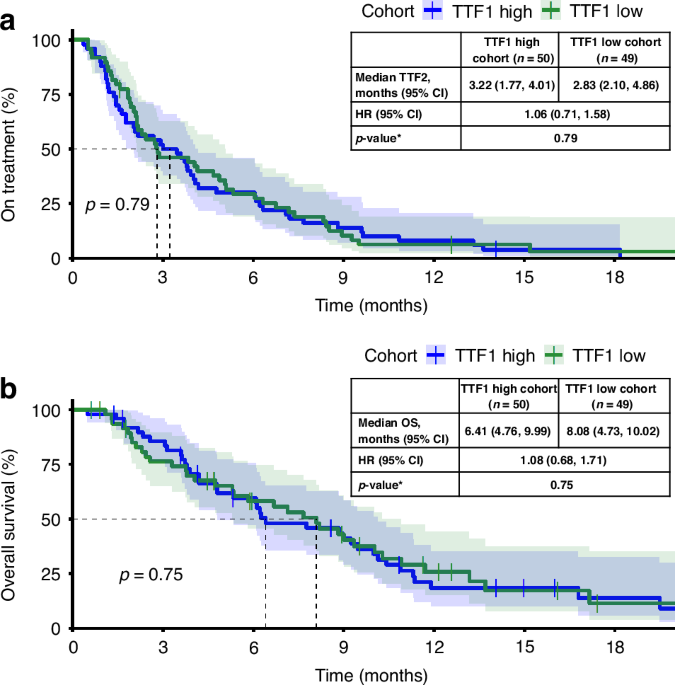
Baseline parameters significantly associated with TTF2 and OS included neutrophil and leukocyte count, CRP levels, liver metastasis and the elevation of CA 19-9. Additional multivariate modelling included the parameters significantly associated with TTF2 and OS in the univariate analysis: CRP levels and neutrophil/leucocyte count remained associated with both TTF2 and OS with p-values of 0.02 or less. In addition, ECOG PS was significantly associated with OS only, whereas elevated CA 19-9 levels at baseline lost their discriminatory power for TTF2 as well as for OS. Of note, the primary hypothesis, which postulated that time-to-treatment-failure of 1st-line (TTF1) predicts 2nd-line treatment outcome (TTF2), was not confirmed.
Overall, median OS was 7.72 months (95%CI 6.11-9.00) and TTF2 reached 3.71 months (95%CI 2.50-4.11) which is in the same range than in other published phase III trials. During treatment, patients with a CA 19-9 response of >25% had a significantly improved TTF2 and OS (p<0.001). The discrimination started at two months with a subsequent additional OS of 8.22 months in responders vs. 4.41 months in non-responders.
Therefore, patients with a normal leucocyte count, low CRP levels and ECOG PS of 0 or 1 have a high chance to benefit from 2nd line chemotherapy, irrespective of the duration of 1st line treatment or elevated CA 19-9 levels. In fact, in patients with increased CA 19-9 levels, start of 2nd line treatment should not be withheld, but rather used for longitudinal assessment. CA 19-9 dynamics after two months or more may help to decide if the potential benefit of chemotherapy still outweighs the efforts.
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in