Chromosome 9p trisomy increases stem cells clonogenic potential and fosters T-cell exhaustion in JAK2-mutant myeloproliferative neoplasms
Published in Cancer

Myeloproliferative Neoplasms (MPNs) are clonal hematological disorders originating from hematopoietic stem cells (HSCs). MPN pathogenesis is marked by the mutually exclusive acquisition of the so-called “driver” mutations affecting JAK2, CALR or MPL genes, all leading to molecular defects in the JAK/STAT pathway and the subsequent overproduction of myeloid differentiated cells (1,2). Driver mutations are often accompanied by secondary mutations in genes involved in chromatin remodeling, splicing, cell proliferation and transcriptional regulation (3). In addition to these genetic mutations, cytogenetic abnormalities are frequently observed in MPNs, particularly involving chromosome 9, where the JAK2 gene resides (4).
Chromosome 9p amplification is a somatic event in JAK2-mutated MPN patients
Starting from the routinary diagnostic Next Generation Sequencing (NGS) analysis, we identified a distinct subgroup of MPN patients who displays both the canonical JAK2 p.V617F mutation and the amplification of the same genomic locus. By validating this finding through Multiplex Ligation-Dependent Probe Amplification (MLPA), we discovered that the genomic amplification encompassed the entire short arm of chromosome 9. Consequently, this subgroup was designated as “+9p patients”. Furthermore, we demonstrated that chromosome 9p amplification is a somatic event restricted to cells belonging to the myeloid lineage such as CD34+ stem and progenitor cells, granulocytes and monocytes, thus being specific to the neoplastic clone. Conversely, CD3+ T cells were found to harbor only two copies of chromosome 9p.
Chromosome 9p duplication involves JAK2 mutated alleles
Next, we sought to determine whether the amplification of the JAK2 locus involved the mutated or wild-type allele. To this aim, we performed a clonal hierarchy study of +9p patients through single-colony genotyping by droplet digital PCR (ddPCR) and single-cell genomics analysis. Both techniques highlighted that JAK2V617F acquisition precedes chromosome 9p duplication. Additionally, we demonstrated that the amplification frequently involves the JAK2 mutated allele, resulting in a cell clone harboring three JAK2 alleles, two of which carrying JAK2 mutation. This genomic asset was the most common among these patients.
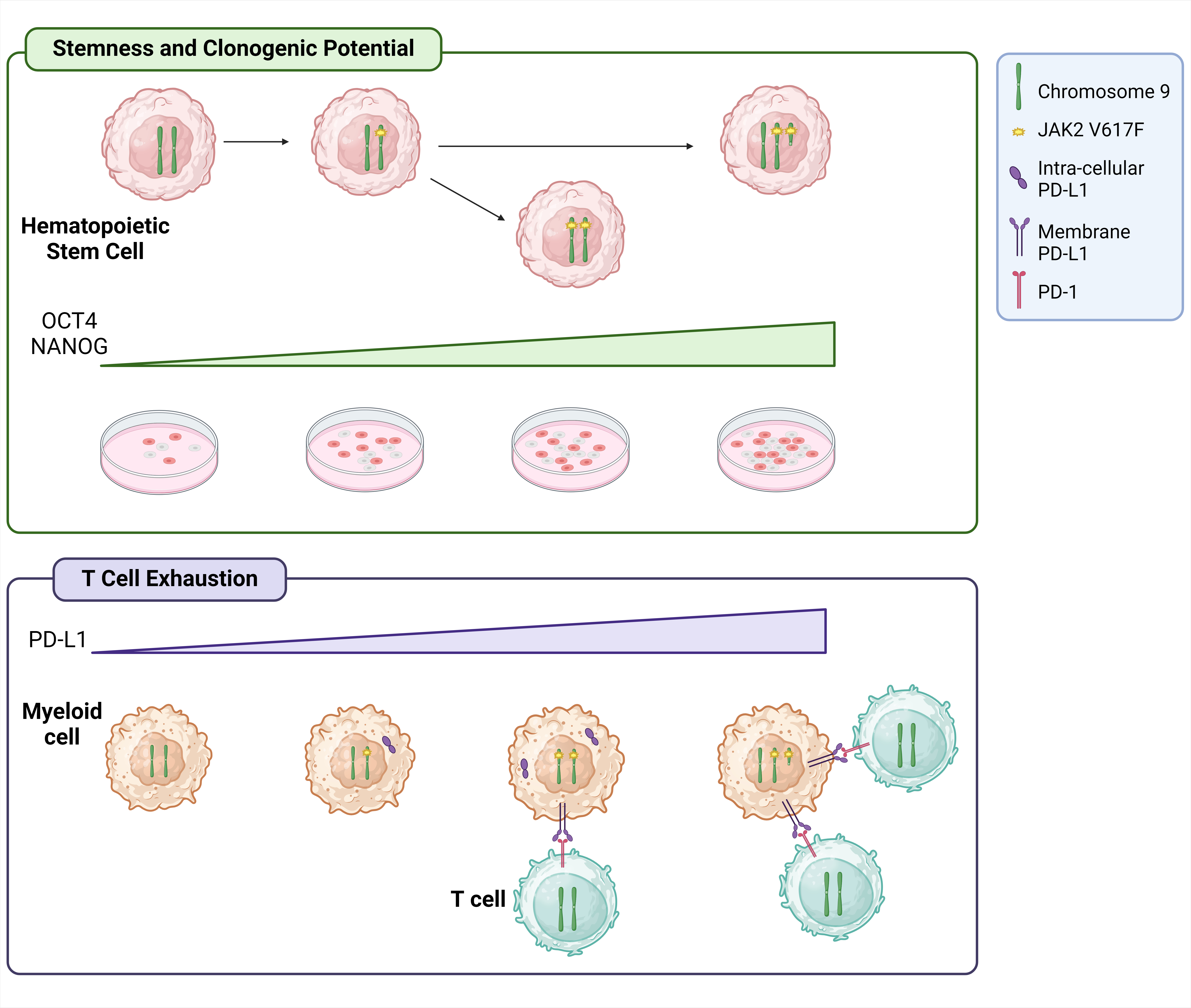
PD-L1 surface expression in +9p monocytes leads to increased T cell exhaustion in +9p MPN patients
To further investigate the role of chromosome 9p amplification in MPN, we examined other genes located on this chromosome. Notably, among these we observed that CD274 gene, encoding Programmed death-ligand 1 (PD-L1) protein, is overexpressed in CD34+ cells, granulocytes and monocytes from +9p patients compared to diploid JAK2-mutated MPN patients. PD-L1 protein is involved in the PD-1/PD-L1 immune evasion axis, often activated in neoplastic contexts (5).
To delve deeper into the role of PD-L1 upregulation in +9p patients, we demonstrated that PD-L1 protein localization is primarily cytoplasmic in heterozygous and homozygous JAK2-mutated patients, whereas it localizes exclusively on the plasma membrane in +9p patients. This finding led us to speculate whether an increase in PD-L1 cell membrane localization may contribute to immune surveillance impairment in this subgroup of patients. Indeed, flow cytometry analysis confirmed that +9p patients display a significantly higher frequency of CD3+/CD8+/CD57-/PD-1+ exhausted T cells compared to JAK2V617F-heterozygous and -homozygous MPN patients, as well as healthy donors (HDs). Additionally, other inhibitory receptors such as CTLA-4, LAG-3, CD244 and TIM-3 were found to be overexpressed in +9p patients relative to HDs. Overall, these findings suggest that PD-L1 overexpression at the plasma membrane level of +9p patients’ cells may enhance PD-1 stimulation on T cells, potentially leading to the development of an exhausted T cell phenotype.
Chromosome 9p trisomy enhances the clonogenic potential of CD34+ cells by upregulating POU5F1 and NANOG
Next, we focused on the study of the impact of chromosome 9p duplication on the HSC function of these patients. Clonogenic assays revealed that CD34+ cells from +9p patients exhibit greater clonogenic potential and a more primitive phenotype compared to those from diploid 9p patients. As previous results by Almozyan et al demonstrated that PD-L1 expression sustains the stemness of breast cancer cells via AKT signaling by upregulating POU5F1, encoding OCT4 protein, and NANOG (6), we wondered if PD-L1 overexpression could impact on stemness and clonogenicity of +9p CD34+ cells.
To explore this further, we investigated whether AKT signaling could contribute to stem cell maintenance in +9p cells by testing CD34+ cells sensitivity to AKT inhibition. Clonogenic assays and single-colony genotyping through ddPCR demonstrated that +9p CD34+ cells were more sensitive to AKT inhibition than diploid cells within the same patient.
We further demonstrated that CD34+ cells from these patients display higher levels of POU5F1 and NANOG compared to JAK2-heterozygous and homozygous diploid patients. This finding was also confirmed in an intra-patient study, as single-colony genotyping associated with gene expression analysis on +9p patients showed that CD274, POU5F1 and NANOG expression levels are significantly higher in triploid cells carrying two mutant JAK2 copies compared to colonies with other genetic profiles within the same patient. Furthermore, POU5F1 and NANOG silencing in CD34+ cells resulted in the decrease of +9p colonies within +9p patient subgroup, reinforcing the notion that PD-L1/AKT/OCT4-NANOG axis plays a role in maintaining the clonogenic potential of +9p CD34 cells.
Conclusions
Overall, this work enabled us to identify and characterize a novel subgroup of MPN patients carrying both the JAK2 driver mutation and chromosome 9p amplification (Figure 1). Our findings demonstrate that in this condition 9p duplication is linked to the increased activation of PD-1/PD-L1 axis, leading to immune escape due to an exhausted T cell phenotype. Additionally, CD34+ cells from these patients exhibit a greater clonogenic potential and a more primitive phenotype compared to diploid JAK2-mutated MPN patients, likely resulting from the upregulation of OCT4 and NANOG through AKT signaling mediated by PD-L1 overexpression. As a whole, this work underscores the importance of an in-depth molecular characterization of oncologic patients. This personalized approach will lead to the identification of pathogenic mechanisms that can be targeted by novel and tailored therapeutic approaches.
References
- Grabek J, Straube J, Bywater M, Lane SW. MPN: The Molecular Drivers of Disease Initiation, Progression and Transformation and their Effect on Treatment. Cells. 2020 Aug;9(8):1901.
- Kralovics Robert, Passamonti Francesco, Buser Andreas S., Teo Soon-Siong, Tiedt Ralph, Passweg Jakob R., et al. A Gain-of-Function Mutation of JAK2 in Myeloproliferative Disorders. N Engl J Med. 2005;352(17):1779–90.
- Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017 Feb 9;129(6):667–79.
- Dunlap J, Kelemen K, Leeborg N, Braziel R, Olson S, Press R, et al. Association of JAK2 Mutation Status and Cytogenetic Abnormalities in Myeloproliferative Neoplasms and Myelodysplastic/Myeloproliferative Neoplasms. Am J Clin Pathol. 2011 May 1;135(5):709–19.
- Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms Controlling PD-L1 Expression in Cancer. Mol Cell. 2019 Nov 7;76(3):359–70.
- 6. Almozyan S, Colak D, Mansour F, Alaiya A, Al-Harazi O, Qattan A, et al. PD-L1 promotes OCT4 and Nanog expression in breast cancer stem cells by sustaining PI3K/AKT pathway activation. Int J Cancer. 2017 Oct 1;141(7):1402–12.
Follow the Topic
-
Leukemia

This journal publishes high quality, peer reviewed research that covers all aspects of the research and treatment of leukemia and allied diseases. Topics of interest include oncogenes, growth factors, stem cells, leukemia genomics, cell cycle, signal transduction and molecular targets for therapy.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in