Core–Shell IrPt Nanoalloy on La/Ni–Co3O4 for High-Performance Bifunctional PEM Electrolysis with Ultralow Noble Metal Loading
As green hydrogen scales toward gigawatt production, proton-exchange-membrane water electrolysis (PEMWE) is throttled by scarce, high-loading precious-metal catalysts. Now a Shanghai Jiao Tong University-led team (Profs. Fuqiang Huang, Wenjiang Ding & Lina Chong) reports a record-breaking bifunctional catalyst that slashes iridium and platinum use by >70 % while delivering multi-year durability in a real PEMWE cell.
Why Low-PGM Bifunctional Catalysts Matter
- Cost Bottleneck: State-of-the-art anodes need 2–4 mg cm-2—far above the DOE 2026 target of <0.5 mg cm-2.
- Performance Gap: Pt is unrivalled for HER but oxidizes to insulating PtO2 under OER conditions; Ir resists corrosion but is HER-poor.
- Manufacturing Simplification: One catalyst for both electrodes halves coating steps, spare-part inventory and stack cost.
Innovative Design & Features
- Core–Shell IrPt Nanoalloy (2 nm): Metallic IrPt core guarantees conductivity; amorphous IrPtO shell supplies abundant *OH/*O binding sites.
- La/Ni-Co3O4 Hierarchical Support: 144 m2 g-1 BET, 5.5 nm mesopores and 30 % oxygen vacancies accelerate water access, gas release and electron transfer.
- Bi-Nuclear OER Pathway: Adjacent *O on Ir–O–Pt couple directly to O2, skipping high-energy *OOH and avoiding lattice-oxygen loss.
- Volmer–Tafel HER: 26 mV dec-1 Tafel slope on Ir–O–Pt delivers Pt-like kinetics at 1/8 the Pt loading.
Applications & Future Outlook
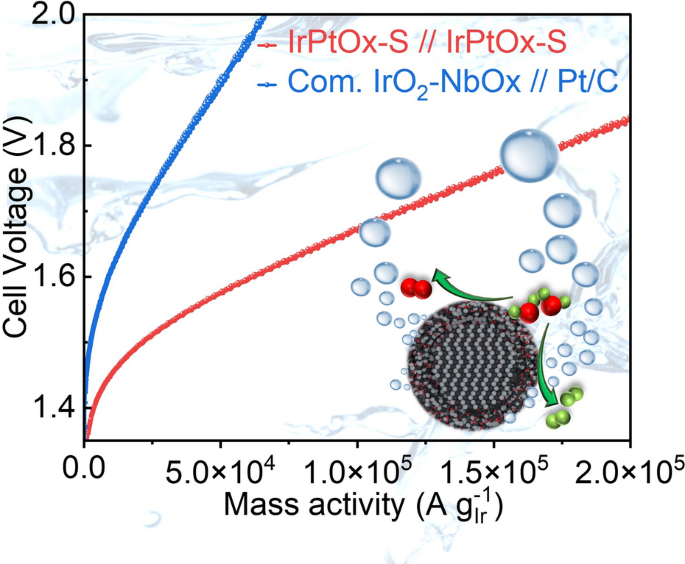
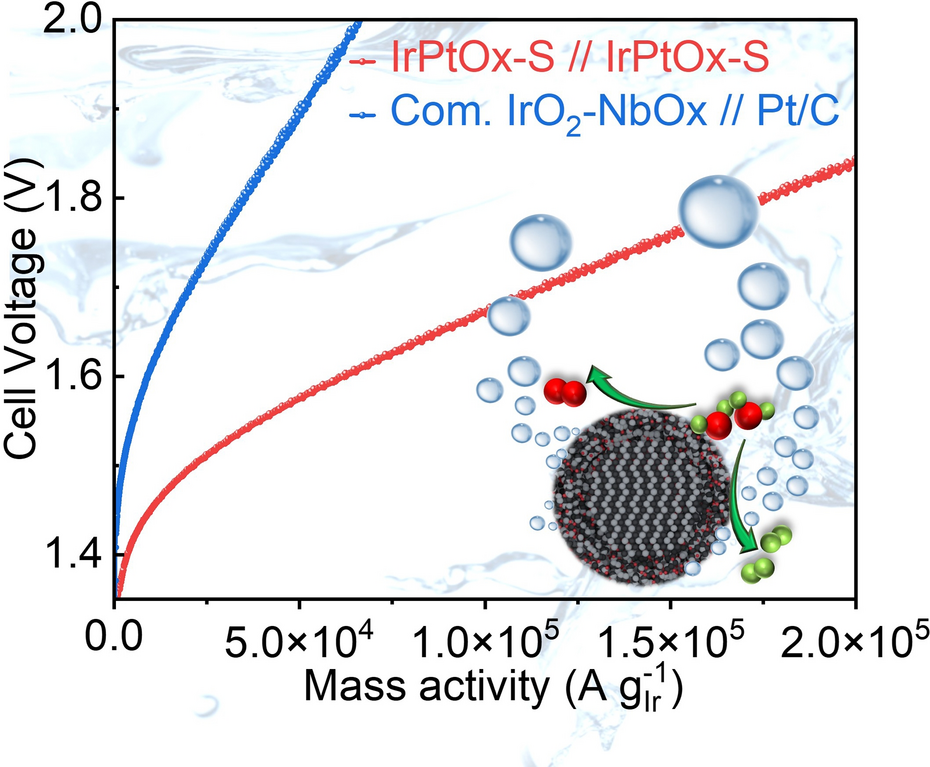
- Real-Cell Validation: Membrane-electrode assemblies with 0.075 mg cm-2 anode + 0.075 mg cm-2 cathode reach 2 A cm-2 at 1.72 V and 72 % electrical efficiency—outperforming commercial 0.2 mg // 0.3 mg benchmarks.
- 646 h Continuous Operation: Degradation only 5 µV h-1; projected lifetime >34 000 h under DOE protocol.
- Green-H2 Cost Roadmap: Catalyst cost falls from $25.2 to $7.9 kW-1; next targets are 1 A cm-2 at 1.55 V and roll-to-roll CCM coating for 1 MW stacks.
This work proves that sub-0.1 mg cm-2 PGM loadings can still unlock multi-ampere electrolysis, offering an immediately translatable route to affordable, grid-scale green hydrogen. Stay tuned for pilot-scale stack tests from the Chong–Huang–Ding joint laboratory at SJTU!
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in