Cytoprotective and stress-free single cell cloning of human iPSCs
Published in Protocols & Methods

Human induced pluripotent stem cells (hiPSCs) can be generated from healthy and diseased individuals and hold tremendous potential for biomedical research and clinical applications. The unlimited self-renewal and multi-lineage differentiation potential of hiPSCs can produce large numbers of well-defined functional cell types. Ideally, in genetic diseases where cells are damaged or non-functional, patient-derived iPSCs would be used to correct underlying genetic defects and then used for disease modeling, drug discovery, and cell therapies. Gene and cell therapies provide unprecedented opportunities for personalized medicine. Just imagine if the power of genome engineering with CRISPR-Cas9 and the iPSC technology could be combined to establish clonal cell lines from a single cell in a quick, safe, and cost-efficient fashion. Based on recent advances, this could soon become a reality in many laboratories.
Human pluripotent stem cells are sensitive cells and require special cell culture conditions for optimal growth. Perturbations to the microenvironment and loss of cell-cell and cell-matrix contact quickly activate a complex cascade of events that lead to poor cell survival. It has been known for many years that routine cell passaging will lead to massive cell death. Scientists typically try to overcome this challenge by seeding more cells or using chemical inhibitors of the ROCK kinase pathway. Although an important advance for the field (Watanabe et al., 2007), inhibition of ROCK kinase 1/2 alone does not protect hiPSCs from several stress mechanisms that otherwise compromise cellular homeostasis, structure, and function as we previously reported (Chen et al., 2021).
To address this need, we performed an unbiased and high throughput screen of 16,000 small molecules across various concentrations, cell densities, and combinations. Through combinatorial screening, we identified a four-component small molecule cocktail that markedly reduced cellular stress and improved cell survival of hPSCs. This small molecule cocktail, which we named CEPT (an acronym for chroman 1, emricasan, polyamines, and trans-ISRIB), is fast acting and has unique cytoprotective properties, thereby inhibiting multiple cell stress mechanisms within the first minutes after single cell dissociation (see Tristan et al., 2022). These include the inhibition of detrimental cell contractions, cell membrane blebbing, abnormal nuclear morphologies, and DNA damage. CEPT also minimizes oxidative stress by maintaining high glutathione levels, avoids the activation of the integrated stress response, and promotes protein synthesis. By maintaining the structural and functional integrity of dissociated cells, CEPT also promotes cell attachment to coated culture plates more efficiently. Importantly, in independent and reproducible experiments using multiple methods and assays, we show that CEPT is superior to Y-27632 and other commercially available reagents (CloneR, RevitaCell, SMC4), and provides superior cytoprotective effects to stem cells at a fraction of the cost (Chen et al., 2021).
In the present study, we expanded on our earlier findings and provide a practical and user-friendly protocol for single cell cloning using CEPT and gentle microfluidic cell dispensing that is amenable for high-throughput experiments in 96- and 384-well plates. This advanced protocol serves as a versatile platform that safeguards the viability and fitness of single cells providing new opportunities for the establishment of clonal cell lines, gene editing, and many other downstream applications.
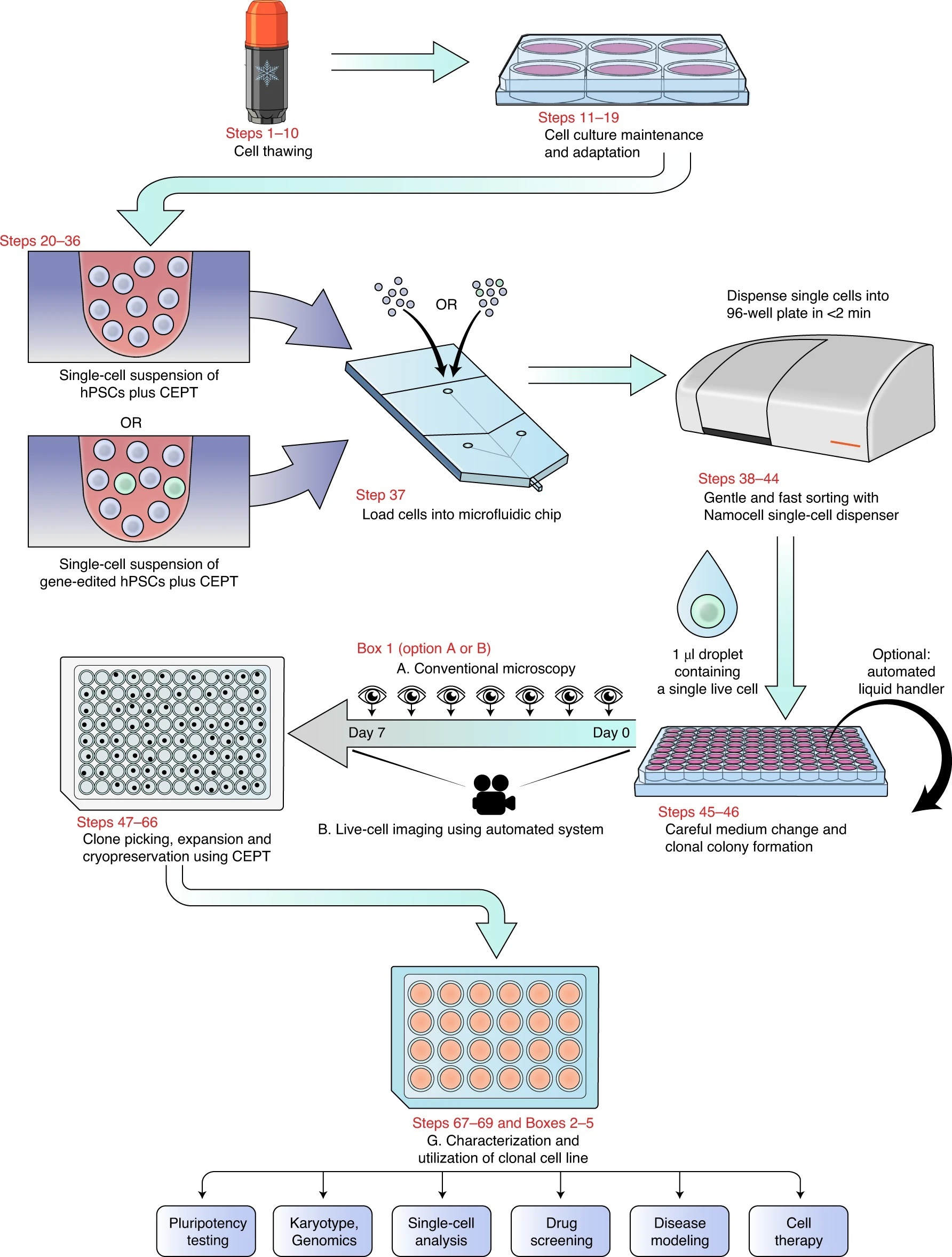
Briefly, hiPSCs are routinely cultured in feeder-free chemically defined E8 medium using the CEPT cocktail for 24 hours at every passage. The cytoprotective effects of CEPT ensure high cell quality and optimal growth conditions of genetically stable cell lines. To demonstrate the advantages of CEPT for single cell cloning, we performed various comparisons with Y-27632, CloneR, and different cell sorting methods including FACS-based cell sorting system (FACSAria III from BD Biosciences) and image-based single cell printing (Cytena SCP from Molecular Devices), and microfluidic cell dispensing (Hana from Namocell). CEPT proved to be the most efficient in all cases. For instance, single cell cloning efficiencies with CEPT were improved 6-fold when compared to Y-27632 and 1.5-fold when compared to CloneR. Notably, these studies established that CEPT in combination with fast, gentle, and microfluidic cell dispensing was particularly user-friendly.
The instrument setup time for Hana is less than 3 minutes, and it dispenses cells at low pressure (less than 2 psi) in 1-2 minutes for a 96-well plate, which greatly reduces mechanical stress and cell damage as compared to traditional FACS instruments. Furthermore, the single cell suspension can be stained with Calcein AM stain to dispense only live cells at a one-cell-per-well condition. Other beneficial factors for single cell cloning were the use of laminin 521 and StemFlex media, which allows performing the first medium change at 72 hours and avoids cell stress associated with automated media changes at earlier time points. Typically, after 7-10 days clonal colonies are formed and can be passaged with CEPT to quickly establish stably growing clonal cell lines. Despite the well-known variability across cell lines, cloning efficiencies for some cell lines were up to 80% (e.g., WA07, WA09, LiPSC-GR1.1, GM25256) in the presence of CEPT. Newly established cell lines can be characterized by using various methods, including expression of pluripotency markers, surface markers, normal cell growth, differentiation potential, karyotype analysis, and more in-depth analyses (e.g., whole exome sequencing).
In summary, we established a single cell cloning method that is based on practical considerations and an improved understanding of the complex interconnected stress mechanisms that hiPSCs will frequently activate in the absence of CEPT. Therefore, utilizing the cost-efficient CEPT small molecule cocktail and stress-free single cell cloning may become a standard approach for establishing next-generation iPSC lines and genome engineering.
Link to full text: https://rdcu.be/cXTj2
References:
Watanabe, K., Ueno, M., Kamiya, D., Nishiyama, A., Matsumura, M., Wataya, T., Takahashi, J.B., Nishikawa, S., Nishikawa, S-I., Muguruma, K. & Sasai, Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25, 681-686 (2007).
Chen, Y., Tristan, C.A., Chen, L., Jovanovic, V., Malley, C., Chu, P-H., Ryu, S., Deng, T., Ormanoglu, P., Tao, D., Fang, Y., Slamecka, J., Hong, H., LeClair, C.A., Michael, S., Austin, C.P., Simeonov, A. & Singeç, I. A versatile polypharmacology platform promotes cytoprotection and viability of human pluripotent and differentiated cells. Nat. Methods. 18, 528-541 (2021).
Follow the Topic
-
Nature Protocols

This journal publishes secondary research articles and covers new techniques and technologies, as well as established methods, used in all fields of the biological, chemical and clinical sciences.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in