Does the anomalous neurogenesis observed in autism serve as a causative factor?
Published in Neuroscience
Our journey into the world of neural stem cells (NSCs) and autism began with a simple but challenging question: “Does the anomalous neurogenesis observed in autism serve as a causative factor? or is it a consequence of the disorder itself?” This puzzle lingered in our lab discussions for years, fueled by intriguing parallels between stem cell quiescence and neurodevelopmental phenotypes in ASD models. The tipping point came when we started noticing recurrent links between ARID1B mutations and altered NSC states—not just in mice, but also in human patients and organoid systems.
The turning point came when we witnessed autism-like behaviors emerging in mice after inducing ARID1B conditional knockout only in adult NSCs—a moment that was both surprising and deeply moving for our team. Building on this, we discovered that inhibiting H3K27me3 could reverse these autism-like symptoms. This compelling evidence showed that modulating the dormancy of qNSCs can directly control social behavior, transforming our understanding of autism’s neurobiological roots. Also, as we extended our work to brain organoids and patient-derived stem cells, the parallels grew: whether genetic (ARID1B, BCL11A, SMARCC2) or sporadic ASD cases, dormant qNSCs were a common thread.
Additionally, we was deeply impressed to observe that autism-related behavioral changes were clearly manifested not only in juvenile animals but also in adults. Previously, autism was generally considered a childhood disorder. However, through this study, we found that genetic manipulation of NSCs in the adult brain could induce social deficits and other autism-like behaviors even in mature animals. This result challenges traditional views about the timing of autism onset and suggests that genetic and epigenetic changes may trigger autism symptoms later in life. As a researcher, directly witnessing adult-onset autism-like traits broadened my perspective on the nature of neurodevelopmental disorders and highlighted new therapeutic possibilities for adult cases.
Key Takeaways and Implications
Our central finding is that aberrant regulation of qNSCs could represent a convergent mechanism in autism pathogenesis across diverse models—genetic, idiopathic, and even adult-onset cases. This suggests that, despite the diverse spectrum of symptoms and origins in autism, there may exist a unified gateway mechanism through which ASD phenotypes emerge—and targeting this pathway could provide a promising therapeutic approach. Thus, reversing qNSC dormancy, whether genetically or pharmacologically, could alleviate core ASD phenotypes. Moving forward, we are excited to pursue patient-specific analyses and longitudinal studies, aiming to correlate qNSC dynamics directly with autism severity and clinical subtypes. Our hope is that sharing these behind-the-scenes observations sparks broader interest and collaboration in exploring the “quiet side” of neural stem cells—and maybe, just maybe, leads to effective interventions for ASD.
Follow the Topic
-
Molecular Psychiatry

This journal publishes work aimed at elucidating biological mechanisms underlying psychiatric disorders and their treatment, with emphasis on studies at the interface of pre-clinical and clinical research.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcement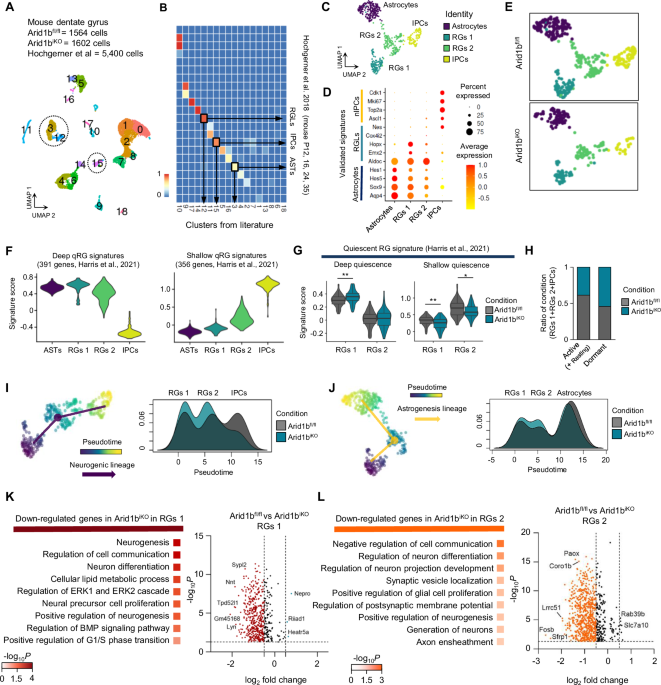

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in