Designing a Sulfur Vacancy Redox Disruptor for Photothermoelectric and Cascade-Catalytic-Driven Cuproptosis–Ferroptosis–Apoptosis Therapy
Published in Chemistry, Materials, and Immunology
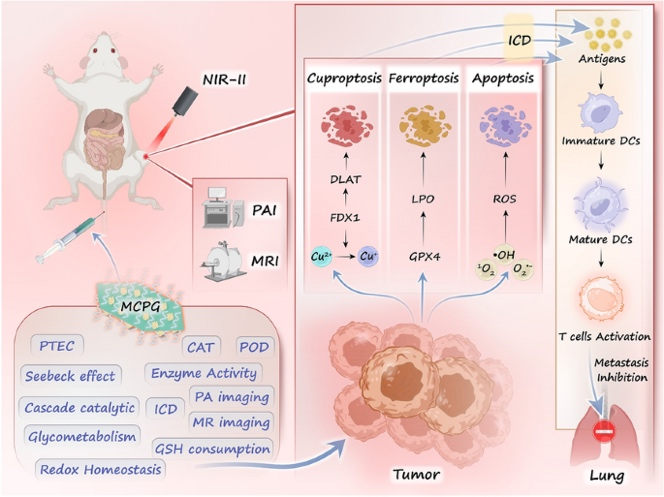
As cancer evolves, the demand for intelligent therapeutics that integrate energy conversion, metabolic interference, and immune activation intensifies. Now, researchers from Harbin Engineering University and Harbin Normal University, led by Professor Piaoping Yang, Professor Lili Feng, and Professor Wei Guo, have delivered a comprehensive study on biodegradable Cu2MnS3-x-PEG/glucose oxidase (MCPG) nanosheets that realize triple-modal cell death. This work offers a blueprint for next-generation nanotherapies that break the “resistance ceiling” of single-mechanism treatments.
Why MCPG Matters
- Energy Conversion: MCPG harvests 1064 nm NIR-II photons, creates a local temperature gradient, and converts heat into electricity via the Seebeck effect, powering in-situ redox catalysis.
- In-Memory Catalysis: Sulfur vacancies and Mn doping act as active sites that continuously replenish H2O2 and O2, sustaining cascade ROS production without external reagents.
- Triple Cell-Death Engine: Simultaneous cuproptosis, ferroptosis, and apoptosis bypass tumor defense pathways and elicit immunogenic cell death with < 1 W cm-2 laser power.
Innovative Design and Features
- Nanosheet Architecture: 2-D ultrathin Cu2MnS3-x-PEG (≈ 4 nm thick, 80 nm lateral) synthesized via one-pot hydrothermal route; PEGylation confers −16 mV ζ-potential for prolonged circulation.
- Vacancy Engineering: Mn doping lowers sulfur-vacancy formation energy from 1.16 eV to 0.80 eV, elevating POD-/CAT-like activity (Vmax 6.9 × 10-8 M s-1, Km 19.7 mM).
- GOx Immobilization: 24.6 % enzyme loading; glucose oxidation provides on-site H2O2, while O2 generation relieves hypoxia, forming a self-amplifying catalytic loop.
- Thermoelectric Performance: p-type conductivity 95 S cm-1, Seebeck coefficient 14 µV K-1, figure-of-merit ZT = 0.0035 at 375 K—sufficient for mild hyperthermia-driven electric biasing.
Applications and Future Outlook
- Multimodal Imaging: Integrated Cu2+ enables T1-MRI (r1 = 0.88 mM-1 s-1) and NIR-II photoacoustic imaging, offering real-time visualization of tumor accumulation (peak at 12 h p.i.).
- Digital Logic of Cell Death: GSH depletion, GPX4 inhibition, DLAT aggregation, and ROS burst are sequentially triggered, providing a programmable “AND-gate” for cancer-selective toxicity.
- Immuno-Oncology: HMGB1 release and DC maturation (CD80+/CD86+ up 2.5×) generate systemic immunity that suppresses lung metastasis by 90 %.
- Challenges and Opportunities: Further studies will focus on large-animal toxicology, scalability of vacancy-rich ternary sulfides, and exploration of low-temperature thermoelectric biasing for deep-tissue lesions.
This comprehensive study delivers a roadmap for integrating photothermoelectric physics, defect engineering, and metabolic intervention in one nanoplatform. It underscores the importance of interdisciplinary collaboration among materials science, catalysis, and tumor immunology to propel the field forward.
Stay tuned for more groundbreaking work from Professor Piaoping Yang’s team at Harbin Engineering University!
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcement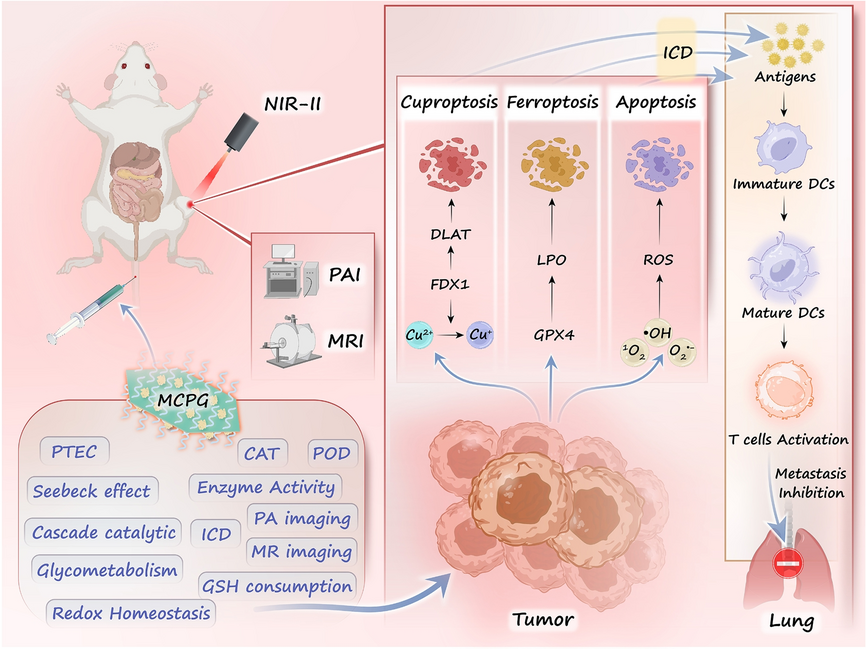





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in