Dipole-driven interlude of mesomorphism in polyelectrolyte solutions---Long ignored, dipole-dipole interactions give life its shape
Published in Chemistry
The paper "Dipole-driven interlude of mesomorphism in polyelectrolyte solutions" has been published in the Proceedings of the National Academy of Sciences, 2022, 119 (40) e2204163119.
The link can be found here: https://www.pnas.org/doi/full/10.1073/pnas.2204163119
Traditionally, scientists have understood charged polymer chains as being composed of smaller, uniformly charged units. Such chains, called polyelectrolytes, display predictable behaviors of self-organization in water: they will repel each other because similarly charged objects don’t like to be close to each other. If you add salt to water containing polyelectrolytes, then molecules coil up, because the chains’ electrical repulsion is screened by the salt.
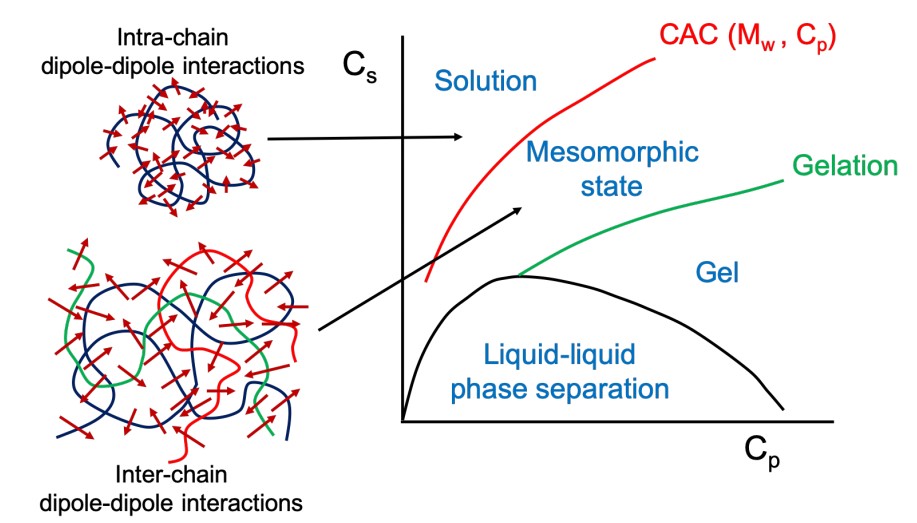
Recently we found that uniformly charged macromolecules or molecules, such as proteins or DNA, that contain a large number of atoms all with the same electrical charge—can self-assemble into very large structures. Liquid-liquid phase separation induced coacervation from a solution of oppositely charged macromolecules at low salt concentration is well known. Here in a polycation-negatively charged organic salt complexation system, we found that dipole-dipole interaction will transform a polycation into a physical polyzwitterion. By tuning the ionic strength, the system exhibits rich phase behavior, including phase separation at low ionic strength and a homogeneous solution at very high ionic strength, with a stable mesomorphic state of monodispersed spherical aggregates with the size around 100-200 nm as an interlude between the two limits. Combing theory and light scattering results, we found that the size of the aggregates depends on the polymer concentration Cp according to the scaling law R~Cp^1/6, and this is due to the inter-chain dipole-dipole interaction. Besides, when the polymer concentration Cp is above a threshold value (with the molar charge ratio of polycation to the oranginc salt fixed), the mesomorphic aggregates disassemble into single chains by a self-poisoning mechanism. Because in addition to part of the organic salt complex with polycation to form dipoles, large amount of excess organic salt will act as salt molecules to self-screen the dipole-dipole interaction. Moreover, in dilute solutions we found isolated polycation chains shrinks upon a decrease in ionic strength, exhibiting “globule-to-coil” conformational transition with increasing ionic strength. Such an “anti-polyelectrolyte effect”, which is a feature of traditional chemical polyzwitterion, is due to the intra-chain dipolar interaction in our system. Combining light scattering results and theory, by tuning ionic strength, polymer concentration, and molar charge ratio, we have systematically studied both the inter-chain and intra-chain dipolar interactions induced physical polyzwitterion system, which exhibits rich phase behavior, which demonstrate that in addition to the electrostatic interactions, dipole-dipole interaction also play an important role in charged polymer systems.
Dipolar polymers are capable of forming complex, self-regulating structures which could be employed in everything from drug-delivery systems to next-generation polymers. We theorize that these dipolar forces in charged macromolecules play a significant role in almost all biological assembly processes, such as the spontaneous birth of membraneless organelles. This work throws new light on one of the fundamental mysteries of life’s processes, or how biological materials know how to self-assemble into coherent, stable structures. The theory changes the paradigm of how we think about these systems, and highlights the unacknowledged role that dipoles play in the self-assembly of biological materials.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in