Discovery of a safe adjuvant to Duchenne Muscular Dystrophy - A journey of 14 years of passion, pursuits & perseverance.
Published in Healthcare & Nursing, Neuroscience, and General & Internal Medicine

On an early summer day in 2009, in Minobu, a small town on the banks of Arakawa River in Yamanashi prefecture, Japan, I met Mr. Takashi Onaka, who was visiting the Nichirenshu grand temple on a pilgrimage. Neither I expected I am set for a long journey of thrills and even risks, nor I realized it was a “ZeitenWende” occasion, then that would lead to the discovery of an adjuvant which after 14 years would be granted an Orphan Disease Designation and Rare Pediatric Disease Designation by the US FDA for treatment of Duchenne Muscular Dystrophy. My objective to meet him was to know about an immune-enhancing beta glucan food supplement, produced by a yeast, whose production method, he had developed, because few years before then, we had started research and technology transfer partnership on autologous immune enhancement therapy using natural killer (NK) cells. As the B-glucan product was in the Japanese market since 1996, I was curious to know how a simple orally consumed food supplement, could boost the human immune system. During the long interaction on that sunny day, Mr. Onaka narrated his story since 1980s. As a natural water processing technopreneur, he had started to interact with Prof. Noboru Fujii in the 80s, who was researching on poly-extremo tolerant organisms and one among them was Aureobasidium Pullulans, a yeast. They hit a eureka moment, when they found that the exo-polysaccharide or exosome like secretion produced by the yeast during a meticulously coordinated culture process was a uniquely structured B-1,3-1,6 glucan. It was a safe orally consumable food product, listed as a food additive in 1996 by the ministry of health and labor welfare (MHLW), Japan. Several clinical and pre-clinical studies they had conducted which were in Japanese and I took time to go through the data, which included prominently the natural Killer cell (NK cell) enhancement in healthy human subjects, followed by control of blood sugar levels on consumption of his B-glucans along with a beneficial reduction in cholesterol levels. As a surgeon by training, to me unless I see, it is hard to believe, and my curiosity to confirm their findings made us conduct two pilot case studies in Type II Diabetes and Dyslipidemia and we repeated the evaluations as the initial data were very significant with the reduction of HbA1C in patients diagnosed with Type II Diabetes Mellitus and reduction in Low-Density Lipoprotein (LDL) cholesterol.
While we were surprised with the reduction in HbA1C in just two months and the triglycerides, with the oral consumption of Beta glucan produced as an exo-polysaccharide by a novel strain AFO-202 of Aureobasidium Pullulans, which we named Nichi Glucan, which were published in 2012 (1) and 2014 (2), we wanted to further evaluate in basic studies their cellular and molecular level beneficial effects. One of the initial few studies we conducted was with in vitro culture of NK cells, by adding these B-Glucan extract to cell culture media, taking help from Dr. Hiroshi Terunuma, Biotherapy Institute of Japan. In the subsequent interaction with the technical team, I came across the terminology “Biological Response Modifier Glucan” (BRMG) wherein they explained on how these B-glucans could be delivering its beneficial effects through the in vivo systems, mentioning about Pathogen Associated Molecular Patterns (PAMP) like properties of this beta glucan resembling or mimicking bacterial Lipopeptide or similar components thereby inducing a natural immunity by triggering the Pattern Recognition Receptors (PRR); all these terminologies were Greek and Latin to me then, by when I had got already entangled in a kind of self-invited “ZeitenWende” trap.
Pillar to post, I got to meet or interact with scientists across domains in biological sciences, which started with Prof. Nobunao Ikewaki of Kyushu Health and Welfare University, Japan who by then had some exciting data published on the immunological properties of Nichi Glucan. The difference between in vitro vs in vivo results somehow was pushing me to expand the horizons. Visited Prof. Jagannatha Rao, who was then in INDICASAT, Panama, an institute working on biodiversity, followed by introduction to Dr. Muralidhar Hegde, who had just moved to Houston Methodist in Texas, USA, and had a track record of research on neurodegeneration and alpha-synucleins and their colleagues had also developed a cell line which on induction could, in in vitro conditions, produce misfolded alpha-synuclein, which was by then a source for developing Parkinson’s disease in vitro models (3). Subsequently we started a collaborative research with Prof. Masaharu Seno, of Okayama University, Japan involved in researching on iPS pathways and carcinogenesis (4). Though the list of collaborations got lengthier, such inter-disciplinary teams’ effort instead of arriving at some answers, only kept on adding more to the list of already existing questions. Though several of those basic studies are continuing even today, two things we could be sure about; One was the safety of the product Nichi Glucan and the other was the involvement of gut microbiome in human health, directly and indirectly, on whom these Beta glucans have a symbiosis. I was worried, that this journey was not going to reach a destination bewildered at the complexities of gut microbiome or the “second genome” and if I want to find one or two species to correlate to them with some criteria of health or disease, it will only amount to try finding a needle in a haystack.
Primarily as a physician, to arrive at a meaningful and explainable solution in terms of a clinical relevance to some illness or condition was always behind me and to make things happen towards that dream, a much stronger involvement with the technical and production team of Beta glucans was indispensable. There came another ZeitenWende wherein the company GN Corporation, we had floated to undertake collaborative research and development of solutions in regenerative medicine had no other choice than becoming a shareholder in the B-glucan manufacturing company M/s Sophy Inc, with their facility in Nyodogawa town of Kochi Prefecture in the Shikoku Island of Japan, making me a technopreneur from a simple clinician-scientist, in 2017-18. Since our involvement, there were two major developments with Sophy Inc. The manufacturing company got GMP certification as per JIHFS norms and launched the new variant beta 1,3-1,6 Glucan produced by N-163 strain of Aureobasidium Pullulans. Till then only the produce of strain AFO-202 of Aureobasidium Pullulans was in the market since 1996, which we tried in metabolic conditions mentioned above. My curiosity of having gone through the basic research data, forced me evaluate how these two products or variants, produced by two different strains of the yeast with unique structures could be biologically value adding to specific conditions.
In a phased manner, we undertook a series of studies in animal models, healthy volunteers and clinical conditions, which unraveled the hidden potentials of the N-163 strain produce, that we named “Neu-REFIX”, leading to the discovery of unmasking its potentials in Duchenne Muscular Dystrophy (DMD):
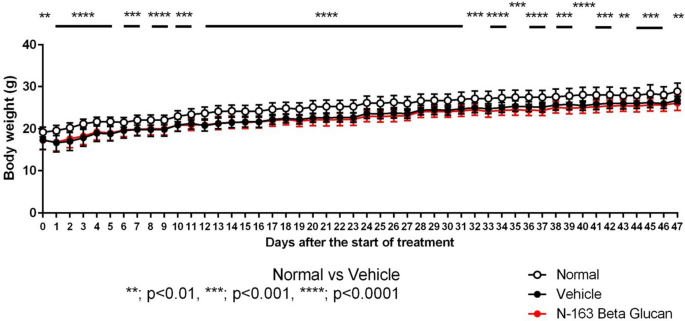
- Non-esterified fatty acid (NEFA) levels in KKAY mice were brought down by Neu-REFIX more than that of Nichi Glucan group fed mice, is an indication of its potentials in reducing inflammation (5).
- Leucocyte to C-reactive Protein Ratio (LeCR) increased in SD rats with Neu-REFIX, proving its potentials an agent of immune modulation (6).
- In an elaborate study in five groups in NASH (Non-Alcoholic Steato-Hepatitis or Steatotic Liver Disease) mice models, Nichi Glucan was found to be a good metabolic regulator and a gut microbiome balancing agent, Neu REFIX acted as an efficient immune modulator (7), along with beneficial reconstitution of gut microbiome, while their combination improved indicators of metabolome and amino acid balancing, and also increased endogenous butyrate (8), that is considered an elixir of healthy long life!
- In healthy male Japanese volunteers, a combination of Nichi-Glucan and Neu REFIX brought down D-Dimer and Inter-Leukin-6, the inflammatory cytokine storm (9).
- During the SARS-COV2 pandemic, we conducted a clinical study in Covid-19 patients in whom Neu REFIX could reduce IL-6 in 30-days (10).
- In another study for 15 days in SARS-COV2, we confirmed the efficacy of Neu-REFIX in 15 days duration in beneficially managing the biomarkers of cytokine storm (11).
Having completed the above six studies, we were sure about the safety along with the anti-inflammatory, anti-fibrotic and immune modulation potentials of Neu REFIX. Now with a safe product possessing specific potentials with us, our search started to find a disease target to which Neu-REFIX would fit in as a suitable and an indispensable therapeutic adjuvant.
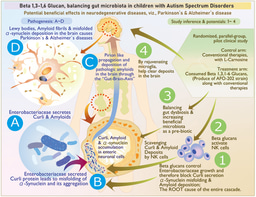
While I had mentioned above about regenerative medicine research and our involvement in immune cell in vitro culture and adaptive immune therapy, a diverse spread of our involvement in various domains in healthcare including medical devices development had its advantage of exposure and connection to experts in various fields making the entire team truly inter-disciplinary. When starting the search for a disease target to match the potentials of Neu REFIX, a major boon to us was Prof. Gary Levy, founding director of multi-organ transplant program of University of Toronto, with whom we had an academic partnership through Training program in regenerative medicine (TPRM) since 2008 (12, 13), which was also his brainchild. His expertise on early detection of post-transplant rejection through appropriate biomarkers, was a great source of learning us on understanding the significance of Neu REFIX’ potentials on immune modulation in a totally safe manner, while the significance of beneficial reconstitution of gut microbiome, mainly the control of harmful ones which not many probiotics or prebiotics have been able to accomplish was something we learnt from Prof. Naoki Yamamoto, an eminent virologist and emeritus professor of Tokyo Medical and Dental University, Japan, whom I knew since the late 90s. With the inputs on all such developments and a survey from Japanese customers who were consuming Nichi Glucans, we had parallelly interacted during the Covid-19 times, with Dr. Kadalraja Raghavan, a developmental pediatrician who had returned back to India after almost two decades of training in Australia and United Kingdom and had completed a pilot clinical study in patients with Autism spectrum disorder in whom constipation got relieved Childhood Autism Rating Scale (CARS) (14) and sleep improved (15) with Nichi glucans which also yielded a beneficial gut microbiome reconstitution (16, 17). Our studies also revealed a significant influence of Nichi Glucans on plasma alpha-synucleins, on which further research efforts with Dr. Rao and Dr. Hegde are under progress (18).
Several sittings of virtual brain-storming we had with Dr. Raghavan and other physicians scientists, and the management of Jaicare Hospital, Madurai, India taking into account all the listed developments of (i) confirmation of safety of these novel beta glucans, (ii) easy palatability by patients of pediatric age group, (iii) potent reduction of inflammation and fibrosis evident by plasma biomarkers in human and animal subjects and (iv) proven anti-fibrotic effects at tissue level in pre-clinical models with liver biopsy along with supporting an eubiosis of gut microbiota. All these deliberations directed us to a consensual decision of Duchenne Muscular Dystrophy as the target disease matching the potentials of Neu-REFIX’, because (i) DMD being a rare or orphan genetic disease doesn’t have any definite cure, (ii) the causative genetic factors are known but due to lack of a definitive treatment, only supportive disease modifying treatment (DMT) approach with steroids and physical therapy was the solace, which again had its range of adverse effects and (iii) exon-skipping therapies approved in the developed nations was a prohibitively expensive affair for patients of Dr. Raghavan. Clinically, the objective of the DMT regimen is to control the inflammation and fibrosis of skeletal muscles thereby preventing the progress of the disease, which was also the potentials on Neu REFIX, though had been proven only in plasma biomarkers and liver biopsy till then, we got approval as per prevailing norms for a short duration of 45-days study clinically in patients with DMD with the help of Jaicare hospital and likeminded organizations offering support to such rare disease patients and would like to make a special mention about the support rendered by Rev. Dr. Francis Xavier, trustee of the JAICARE hospital.
We were astonished to see that, apart from safety in all the patients from the age of 5~20 years, diverse in terms of disease severity, stage of the disease, genetic defect, ambulatory status, both under steroids and otherwise, most of the plasma biomarkers of relevance to inflammation reduced in 45 days in the treatment-arm compared to the control-arm (19). Strikingly, the plasma dystrophin, considered secreted by the vascular smooth muscles and responsible for the basis of pathogenesis of DMD according to the vascular theory (20) increased by 32% along with a beneficial reconstitution of the gut microbiome. Abundance of genus and species of gut microbiota which are considered anti-inflammatory increased those reported to have pro-inflammatory nature decreased (21). Now we had to prove whether the skeletal muscle fibrosis is reduced, which being an invasive procedure, a pre-clinical study was undertaken in MDX mice models with the expert guidance of Dr. Yoshitsugu Aoki of National Center for Neurology (NCNP), Tokyo, Japan (22), a co-developer of several exon skipping therapies (23), some approved and some under clinical trials. His introduction was through the good office Prof. Masaru Iwasaki, Vice-president, University of Yamanashi and also head of Center for Advancement of Clinical Research (CACR). Our study in MDX yielded indisputable evidence confirming the efficacy of Neu-REFIX by a statistically significant reduction of skeletal muscle fibrosis compared to the control group (24). Then we questioned our own selves, whether the reduction in fibrosis and inflammation had got a positive outcome with muscle regeneration. There came the timely collaboration with Prof. Shuji Sakamoto of Kochi University, who evaluated the specimens with more than one parameter and confirmed an enhanced skeletal muscle regeneration in the group of MDX mice that had consumed Neu REFIX (25). Both the reduction of fibrosis and regeneration having proven to be immensely positive, with Neu REFIX, our next clinical study for a longer duration of 180 days in which we evaluated the muscle strength in terms of MRC score, (Medical Research Council), NSAA score (North Star Ambulatory Assessment) and 6MWT score (Six-Minute Walking Test) did improve slightly in just six months, which in other studies with the latest medications have been proven only to decline, however the rate and momentum of decline in those studies being slower or lesser than the control-arm or historical data. When all these studies continuing to prove the safety of the neu REFIX alone or even when consumed along with standard-of-care medications and pre-clinical studies proving the stand-alone efficacy of Neu-REFIX, lately we got another breaking news of several other independent groups around the world proving the correlation of gut microbiome in DMD which was never heard before 2023, and indeed the gut microbiome evaluation in our clinical study subjects revealed an increase of anti-inflammatory species while the pro-inflammatory once declined (26).
When these data as a manuscript, were under peer review in journals, I got a referral through a common acquaintance of a patient with a rare gene defect, somewhat close to DMD, with the pathology centered in the heart. I discussed about the clinical relevance with my long-term acquaintance and cardiologist Dr. Prem Sekar (Kamakshi memorial Hospital, Chennai, India) and one of my mentors in cardiac surgery, Dr. KM. Cherian (Frontier lifeline hospital, Chennai, India), a globally eminent cardiac surgeon, who did the first heart and also heart & lung transplant in India. They both gave their opinion in the background of our data, recommending an investigation on myocardial fibrosis of MDX mice specimens of the heart that had been cryopreserved. Few weeks from then, we got the report confirming the Neu REFIX group with significantly reduced fibrosis of the heart, and the data as a manuscript is now under review (27). Here we learned that the myocardial fibrosis which in DMD occurs at an advanced stage of the disease is also common in the organs that are transplanted, giving us a lead that in fibrosis occurring after organ transplantation, there could be a new application for the Neu-REFIX.
This journey of 14 years since 2009 has happily led to a discovery offering hope of a safe agent to slow down the progress of Duchenne Muscular Dystrophy, that may help prolong the lifespan of the patients affected with this orphan disease, which from now we have to further evaluate in long term studies, and this may even lead to the development of a drug to DMD or in conditions that have common pathogenesis.
Though the journey of thrills, risks and turning points, having completed two clinical studies and several evaluations in pre-clinical studies unmasking the safe and efficacious potentials of Neu REFIX yielding hope to patients with DMD as a discovery and with another study to which grant was sanctioned, I thought it is a happy ending, but the ZeitenWende is seemingly not ending! In the 1980s what started as an exploration of poly-extremo tolerance by Prof. Noboru Fujii having turned on the switch to this discovery of Neu-Refix in DMD, got a shot in the arm with my interaction with Dr. Craig Venter on the 24th October 2023 in Toronto, on the eve of Edogawa NICHE prize lecture. Immediately upon his arrival after five-hour journey from San Diego into Toronto, he took some time to have a chat, following a conversation on Curiosity, intelligence, insanity and their genetic association, he shared with me some excerpts on “The Voyage of the sorcerer II” (28) specially on the findings of the abundance of bacteriophages in crystal clear sea water and their implications, that made me humble on how we are under the control of the microbiome both within and out, in water and in air though I don’t know about that in outer space. After getting to know on his recent initiative of a research on phages for managing the biggest challenge of modern times, the anti-microbial resistance, we are persuaded to revisit our gut microbiome data derived from the pre-clinical and clinical studies to check the influence of Neu-REFIX on endogenous bacteriophages. I would admit that the ZeitenWende continues!
*ZeitenWende is a German word meaning “Turning Point” especially in History or times; This term came across during a discussion when Mr. Daniel Jeyaraj, was in Japan as an intern on inter-disciplinary research, with Dr. Rajappa Senthilkumar (29) and Dr. Leo. M. Jeyaraj (30), joined virtually by Dr. S.P. Preethy (31).
References:
- https://doi.org/10.1155/2012/895370
- https://doi.org/10.3109/19390211.2013.859211
- https://pubmed.ncbi.nlm.nih.gov/31863801/
- https://ssp.jst.go.jp/sns/news/sat/science/201709_2.html
- https://doi.org/10.1007/s40200-022-01170-5
- https://doi.org/10.1016/j.clicom.2021.11.001
- https://doi.org/10.1016/j.jceh.2022.06.008
- https://dx.doi.org/10.1136/bmjgast-2022-000985
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10457473/
- https://doi.org/10.1016/j.biopha.2021.112243
- https://doi.org/10.1101/2021.12.14.21267778
- regenmedcanada.com
- https://www.ncrm.org/feat/tprm/
- https://dx.doi.org/10.1136/bmjno-2021-000203
- https://doi.org/10.1002/brb3.2750
- https://pubmed.ncbi.nlm.nih.gov/36093695/
- https://communities.springernature.com/posts/leveraging-the-gut-microbiome-to-manage-from-neurodevelopmental-to-neurodegenerative-illnesses
- https://pubmed.ncbi.nlm.nih.gov/36310016/
- https://www.sciencedirect.com/science/article/pii/S2667242123000556
- https://www.science.org/doi/10.1126/science.172.3988.1143
- https://doi.org/10.1101/2022.12.09.22283273
- https://www.ncnp.go.jp/nin/guide/r_dna2/greeting_en.html
- https://www.ncnp.go.jp/topics/2022/20220317e.html
- https://www.nature.com/articles/s41598-023-44330-0
- https://doi.org/10.1038/s41598-025-92258-4
- https://doi.org/10.1136/bmjnph-2023-000776
- https://doi.org/10.1101/2023.09.25.559276
- https://www.thevoyageofsorcererii.org/
- https://pubmed.ncbi.nlm.nih.gov/?term=rajappa+senthilkumar
- https://pubmed.ncbi.nlm.nih.gov/?term=jeyaraj+leo
- https://pubmed.ncbi.nlm.nih.gov/?term=preethy+senthilkumar
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Related Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Obesity
Publishing Model: Hybrid
Deadline: Apr 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in