Efficient Neutral Nitrate-to-Ammonia Electrosynthesis Using Synergistic Ru-Based Nanoalloys on Nitrogen-Doped Carbon
As fertilizer demand rises and nitrate pollution spreads, turning waste NO3- into green NH3 has become urgent. Now, researchers from Guizhou University, Hunan Agricultural University and Shanghai University, led by Professor Jili Yuan, Professor Wei Li and Dr Liang Wang, report a selective-etching route to RuM (M = Fe, Co, Ni, Cu) nanoalloys that deliver 100 % Faradaic efficiency for neutral ammonia electro-synthesis at only −0.1 V vs RHE—outperforming most catalysts reported to date.
Why RuM Nanoalloys Matter
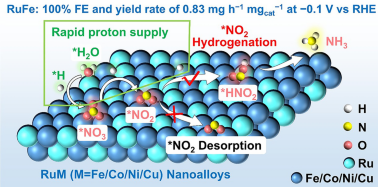
• Energy Efficiency: Alloying shifts the Ru d-band center upward, cutting the *NO2 → *HNO2 barrier to 0.46 eV and suppressing the hydrogen-evolution side-reaction that normally wastes electrons.
• In-Electrode Conversion: The porous nitrogen-doped carbon host ensures rapid proton/electron delivery, enabling 0.83 mg NH3 h-1 mgcat-1 at room temperature without external heat or pressure.
• Circular Nitrogen Economy: When assembled into a Zn–NO3- battery the cathode produces 0.688 mg NH3 h-1 mgcat-1 while delivering 10.16 mW cm-2 power—turning nitrate remediation into a self-powered chemical plant.
Innovative Design and Features
• Nanoalloy Types: RuFe-NC, RuCo-NC, RuNi-NC and RuCu-NC (2–3 nm) are etched in molten urea/NaCl, yielding lattice-contracted (101) planes and uniform metal dispersion verified by HR-TEM, XRD and XPS.
• Functional Support: Chloride-assisted etching creates mesopores (20–50 nm) that triple the electrochemical surface area, while nitrogen dopants anchor alloys and lower the work function for faster electron injection.
• Device Architecture: Drop-cast on carbon paper, the catalysts serve as air-breathing cathodes in H-cells and coin-type Zn batteries, demonstrating scalability from 1 cm2 lab cells to 24 h continuous timers.
Applications and Future Outlook
• Multi-Level Selectivity: RuFe-NC reaches 100 % NH3 FE at −0.1 V, while RuCo-NC maintains 98 % FE after 12 cycles and 96 % in 0.05 M NO3-—a tolerance window critical for real wastewater.
• Digital Logic Gates: The catalyst layer doubles as a low-potential sensor pixel; changes in NH3 FE are converted to voltage signals, offering a new route for nitrate-aware IoT nodes.
• Artificial Nitrogen Fixation: In-situ Raman, EPR and ATR-SEIRAS reveal *HNO2 and *NH2 intermediates, confirming a synergy-driven pathway that can be generalized to Co-, Ni- or Cu-based ternary alloys for urea, hydrazine or C–N bond construction.
• Challenges and Opportunities: The team highlights the need for roll-to-roll etching protocols, membrane-electrode assemblies that separate NH3 from the electrolyte, and life-cycle studies comparing energy input to Haber–Bosch. Future work will explore Ru–Co–Fe trimetallics and AI-guided composition tuning to push onset potentials above 0 V RHE.
This work provides materials chemists, environmental engineers and energy-storage designers with a universal alloying blueprint for turning nitrate waste into carbon-free ammonia while co-generating electricity. Stay tuned for more advances from Professor Jili Yuan, Professor Wei Li and Dr Liang Wang!
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in