Elucidation of radical- and oxygenate-driven paths in zeolite-catalyzed conversion of methanol and methyl chloride to hydrocarbons
Published in Chemistry

To understand why we were particularly interested in studying methyl chloride coupling, we need to return to the start of our journey over 20 years ago with the first contribution about HCl oxidation chemistry on ruthenium oxide.1 The knowledge gathered since then has been pivotal for the next chapter of our story that started in 2016: understanding the halogen-mediated upgrading of natural gas. The concept entails the reaction of a light alkane, such as methane, ethane, or propane, with oxygen and a hydrogen halide, i.e., HCl or HBr, over a catalytic surface to yield the corresponding alkyl halide.2 The latter can be further converted into olefins, aromatics, and oxygenates by using different systems, including zeolites, metal oxides and metalorganic complexes, known to convert similarly structured alcohols, such as methanol and ethanol. This approach has the advantage of maintaining high selectivities (≤ 95%) to the desired product at high methane (≤ 30%) and higher alkanes (≤ 90%) conversion compared to direct oxidation approaches. These high single-pass yields have attracted broad interest, with efforts devoted to demonstrating the viability of the technology for using natural gas as greener feedstock for petrochemicals compared to oil and coal in the transition towards renewables.3
Although chemically similar to the commercially-applied zeolite-catalyzed conversion of methanol to hydrocarbons (MTH), the analogous reaction of methyl chloride to hydrocarbons (MCTH) suffers from lower activity and uncontrolled chain growth, leading to suboptimal catalyst selectivity and stability.4,5 To better understand the differences, we focused on understanding the formation and propagation of C−C bonds in MCTH by combining kinetic analyses and advanced spectroscopic methods. The comparison with MTH as a reference reaction, enables the identification of shared and distinct mechanistic paths. The accurate discrimination of reactive intermediates, often present in low concentrations, remains a longstanding challenge in complex hydrocarbon conversion. For example, methyl radicals and ketene have been predicted to be active intermediates in MTH but never experimentally observed.5,6 Furthermore, even less is known about the origin and nature of coke precursors and how these species evolve into large carbonaceous deposits.
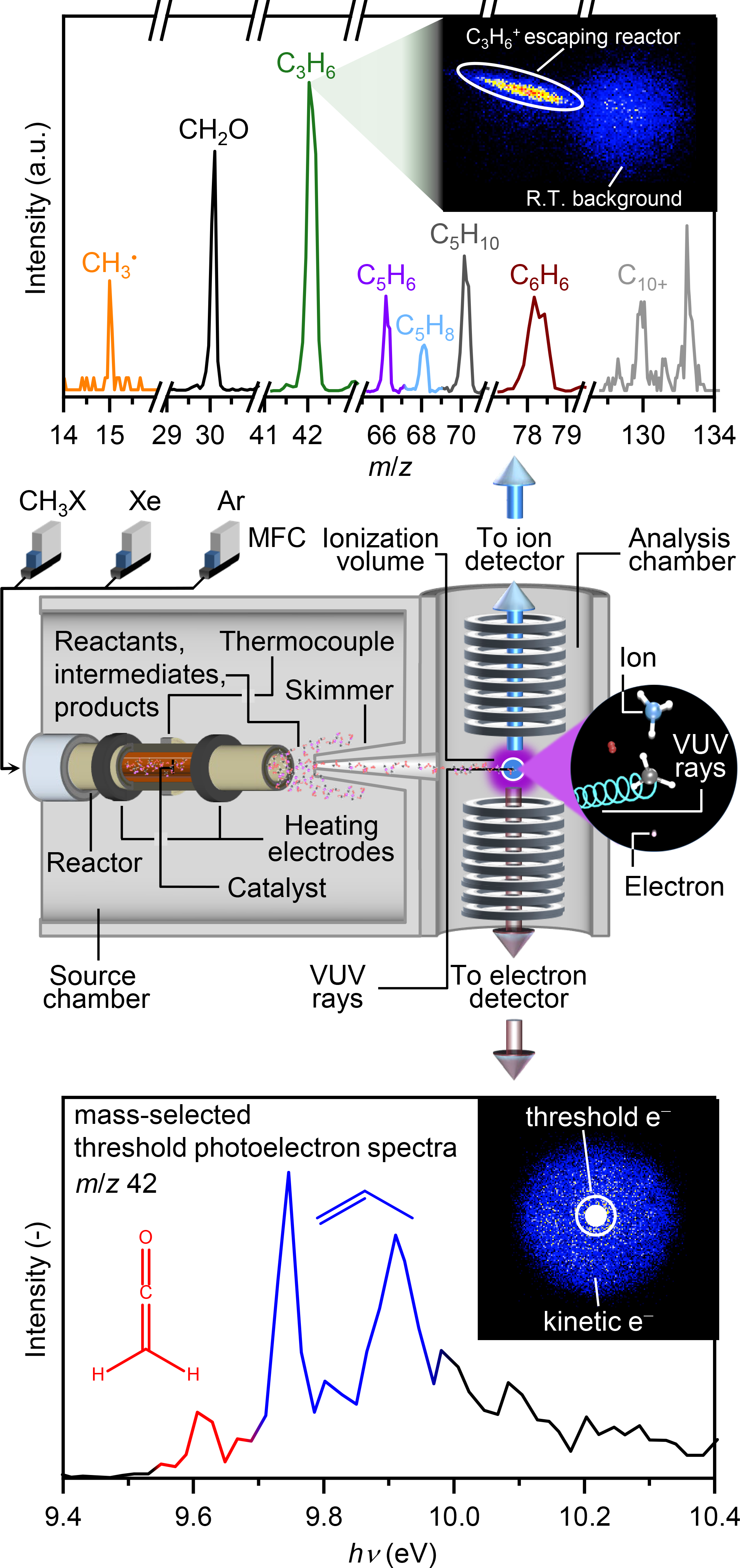
Figure 1. Scheme of the PEPICO reactor setup used for operando isomer-selective detection of short-lived intermediates in this study. The central panel illustrates the gas delivery system, the reactor, the ionization source, and the detectors, while the top and bottom panels depict the typical spectra obtained.
The solution to this challenges was inspired by one of our earlier studies on the mechanism of catalytic alkane oxyhalogenation from 2018, in collaboration with the group of Dr. Andras Bodi and Dr. Patrick Hemberger at the Swiss Light Source (SLS) of the Paul Scherrer Institute in Switzerland. Detailed kinetic analyses had pointed to the co-involvement of both gas-phase and surface-catalyzed pathways, but only using operando photoelectron photoion coincidence spectroscopy (PEPICO), could we detect highly reactive species in the gas phase, confirming the occurrence of both surface and gas-phase steps.7-10 The technique’s sensitivity derives from coupling a heated microreactor and a gas sampling setup to the detection chamber, as depicted in Figure 1. The configuration minimizes the quenching of short-lived species desorbing from the catalyst surface or generated in the gas phase by feed dilution and low pressure inside the source chamber, while maintaining relevant conditions inside the reactor. Briefly, photoelectrons and photoions, generated by ionizing a molecular beam skimmed from the reactor, are separated by an electric field and detected in delayed coincidence. Photoions are analyzed by time-of-flight mass spectrometry, which reveals their mass-to-charge (m/z) ratio, thus indicating the identity of the species (Figure 1, top). Photoelectron signals are processed via momentum imaging and can be related to photoions thanks to the delayed coincidence detection.
Given the potential of operando PEPICO spectroscopy, we decided to apply this technique in MTH and MCTH to gain insights into some of the still unanswered questions in the mechanism of these reactions. After the successful campaign at the SLS, we obtained thousands of spectra after screening different conditions in both MTH and MCTH that had to be extracted, processed, and analyzed, each of which containing multiple species spanning from C1 to C14. The hard work was rewarded when we saw the unique vibrational fingerprints of methyl radicals in MTH and MCTH and ketene in MTH (Figure 2). The observation of ketene was exciting because this species has the same integer mass as major-product propylene, but both could be clearly distinguished due to their different ionization energy (Figure 1, bottom). Furthermore, the study demonstrated that neither oxygen- nor chlorine-containing intermediates and products are formed in MCTH. Accordingly, by comparing the evolution of different hydrocarbons in MTH and MCTH, we could discern the oxygenate-driven and the methyl radical-addition pathways in MTH, revealing key similarities and differences between the two reaction networks (Figure 2).
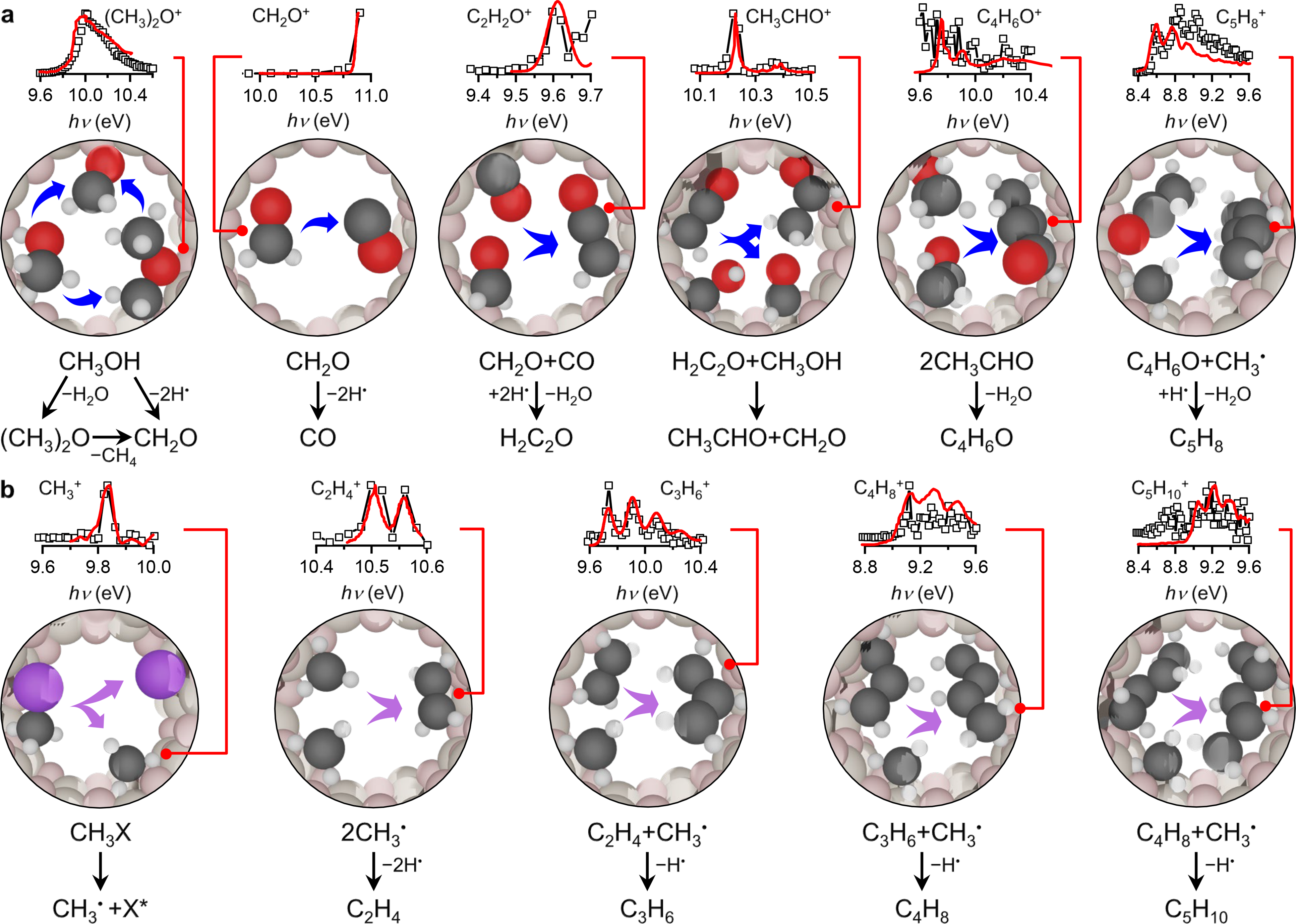
Figure 2. Reaction pathways of catalytic C1 coupling. Proposed reaction network of a, the oxygenate‑driven reaction in MTH and b, the direct CH3• radical-addition pathway in MTH and MCTH to yield C5 intermediates in the micropores of H‑ZSM-5. The insets on top of the molecular schemes show the ms‑TPE (open squares) and reference spectra (solid lines) of the identified species in a, MTH and b, MCTH. The main identified isomers are represented by the molecular models in a,b. Color code: C (grey), O (red), H (white), X (X = OH, Cl; violet).
In addition, operando PEPICO allowed us to identify a wide range of species, up to C14, involved in the generation of carbonaceous deposits. Thanks to their detection, we could propose potential pathways for the formation of polycyclic aromatic compounds. Still, coupling this knowledge with the analysis of deposited carbonaceous species, often restricted to quantitative information on the amount formed within the zeolite pores with little information on the chemical nature, remained a challenge. Since coke species are typically paramagnetic, electron paramagnetic resonance (EPR) spectroscopy can offer valuable insights. We, therefore, teamed up with the group of Prof. Gunnar Jeschke at ETH Zurich, with leading expertise in this field. Due to current limitations in operando EPR,11 we opted for different ex situ continuous and pulsing EPR techniques, which, by operating at low temperatures, have the advantage of high resolution. Notably, the 2D EPR experiments depicted in Figure 3 are of particular relevance as they allowed us to extract a representative molecular structure of the carbonaceous species deposited in the zeolite micropores after MTH and MCTH, closing the gap between coke precursors and deposits.
Understanding how C−C bonds form and propagate into complex species has important practical implications for designing superior catalytic and reactor systems for the MTH and MCTH reactions. Going forward, these techniques provide a valuable platform for gaining insights into other long-standing questions in hydrocarbon functionalization processes, distinguishing spectators from reactive reaction intermediates.
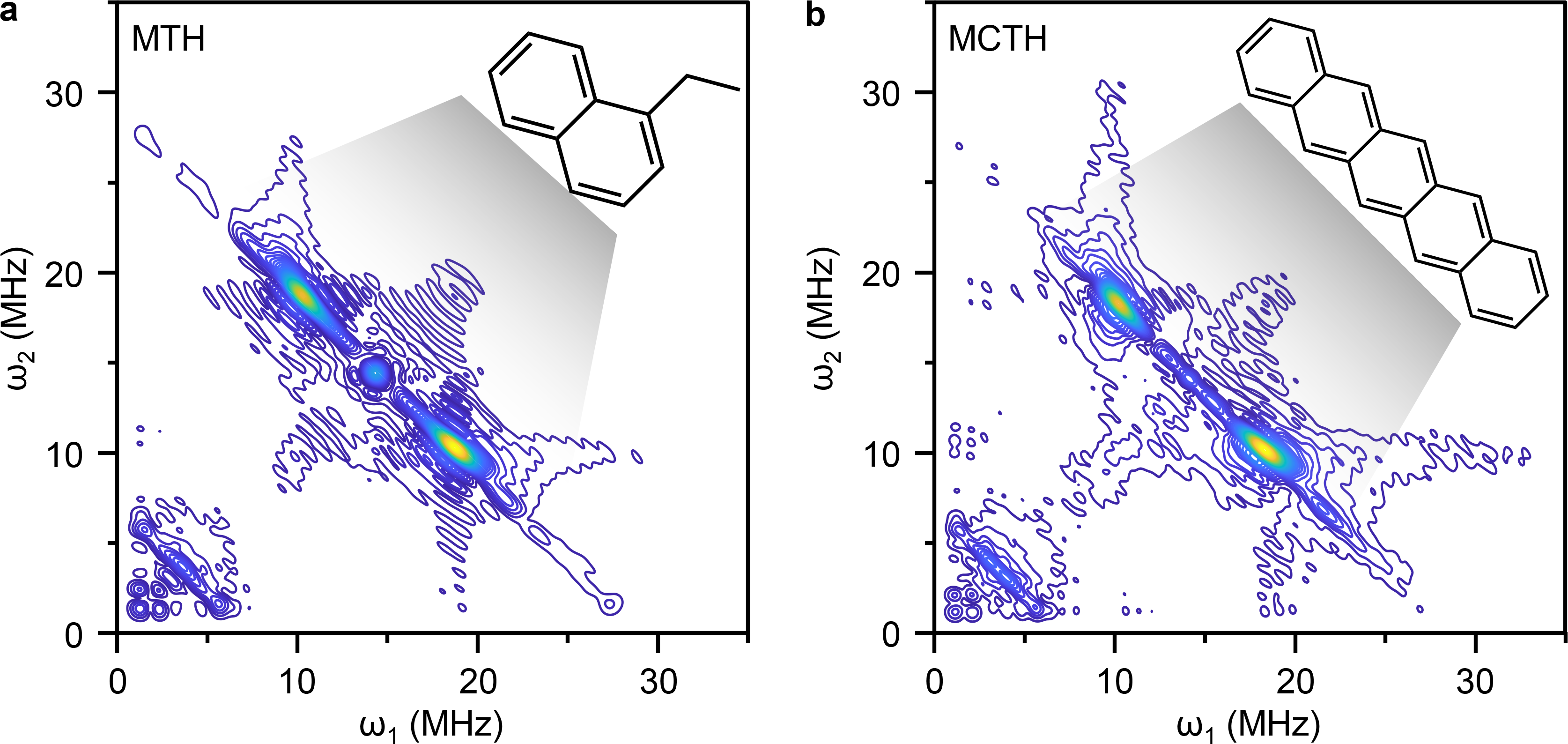
Figure 3. Representative molecular structure of carbonaceous deposits. Weak interaction quadrant of the 2D HYSCORE spectra of H‑ZSM-5 after 2 h on stream in a, MTH and b, MCTH.
References
- López, N., Gómez-Segura, J., Marín, R. P., Pérez-Ramírez, J. Mechanism of HCl oxidation (Deacon reaction) over RuO2. J. Catal. 2008, 255, 29-39.
- Paunović, V., Zichittella, G., Moser, M., Amrute, A. P., Pérez-Ramírez, J. Catalyst design for natural gas upgrading via oxybromination chemistry. Nat. Chem. 2016, 8, 803-809.
- Zichittella, G., Pérez-Ramírez, J. Status and prospects of decentralised natural gas valorisation to energy and energy carriers. Chem. Soc. Rev. 2021, 50, 2984-3012.
- Lin, R.; Amrute, A. P. & Pérez-Ramírez, J. Halogen-mediated conversion of hydrocarbons to commodities. Chem. Rev. 117, 4182-4247 (2017).
- Yarulina, I., Chowdhury, A. D., Meirer, F., Weckhuysen, B. M. & Gascon, J. Recent trends and fundamental insights in the methanol-to-hydrocarbons process. J. Catal. 1, 398-411 (2018).
- Chowdhury, A. D. & Gascon, J. The curious case of ketene in zeolite chemistry and catalysis. Angew. Chem. Int. Ed. 57, 14982-14985 (2018).
- Paunović, V., Hemberger, P., Bodi, A., López, N. & Pérez-Ramírez, J. Evidence of radical chemistry in catalytic methane oxybromination. Nat. Catal. 1, 363-370 (2018).
- Zichittella, G., Scharfe, M., Puértolas, B., Paunović, V., Hemberger, P., Bodi, A. & Perez-Ramirez, J. Halogen-dependent surface confinement governs selective alkane functionalization to olefins. Angew. Chem. Int. Ed. 58, 5877-5881 (2019).
- Zichittella, G., Hemberger, P., Holzmeier, F., Bodi, A. & Perez-Ramirez, J. Operando photoelectron photoion coincidence spectroscopy unravels mechanistic fingerprints of propane activation by catalytic oxyhalogenation. J. Phys. Chem. Lett. 11, 856-863 (2020).
- Hemberger, P., van Bokhoven, J. A., Pérez-Ramírez, J. & Bodi, A. New analytical tools for advanced mechanistic studies in catalysis: photoionization and photoelectron photoion coincidence spectroscopy. Catal. Sci. Technol. 10, 1975-1990 (2020).
- Zichittella, G., Polyhach, Y., Tschaggelar, R., Jeschke, G. & Pérez-Ramírez, J. Quantification of redox sites during catalytic propane oxychlorination by operando EPR spectroscopy. Angew. Chem. Int. Ed. 60, 3596-3602 (2021).
Follow the Topic
-
Nature Catalysis

This journal brings together researchers from across all chemistry and related fields, publishing work on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, incorporating both fundamental and applied studies.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in