Engaging underserved communities in clinical trials
Published in Biomedical Research and General & Internal Medicine

Can you name this boat?

It became a welcome, if slightly unexpected, mascot for the importance of including the public in your research at RDF25, the NHS’s Research & Development conference.
If you’re in the UK, there’s a chance you might recognise this polar research vessel by its official name, RRS David Attenborough. But more likely, you’ll remember it as Boaty McBoatface, the most popular suggestion put forward when the public were asked to vote on names in 2016.
But what does this have to do with clinical trials? This was an example put forward by the NIHR’s Emily Pickering, to illustrate not just the importance of involving patients in clinical research and study design from the very start, but also the importance of being prepared for their ideas to completely change and challenge your perceptions of how the research should be carried out.
While involving patients from the start goes a long way for making sure we get research right first time, the patients taking part as clinical trial participants themselves also need to accurately represent the wider population.
That isn’t currently the case, though. The patients who are most likely to sign up to clinical trials are largely university-educated, middle class, middle-aged, and white, who’ve seen posters in their GP waiting rooms. They’re not a good representation of the breadth of people affected by various health conditions, and might not be the primary target of the condition in focus.
As a real-life example of this, a conference delegate I spoke to pointed out that while there’s a high incidence of Type 2 Diabetes in Northwest England and Scotland, the ‘golden triangle’ of diabetes clinical trials falls within the South East, excluding many of these patients from being involved.
This misrepresentation leaves communities of underserved groups within the population – people who have not been included and considered in clinical trials, and who medical treatment might not be as effective for.
What is an underserved community?
There’s no single definition of an underserved community in the context of healthcare, but this could be considered as any group who is less likely to take part in clinical research.
The importance of including participants who accurately reflect the population is really well reflected in this year’s International Clinical Trials Day theme – “Rethinking Clinical Trials: Inclusivity In Practice” and in Sustainable Development Goals 3 (Good Health and Wellbeing) and 10 (Reduced Inequalities).

Who’s missing from clinical research?
At the moment, communities likely to be missing from clinical research in the UK might include people coming from:
- Backgrounds of economic disadvantage
- Ethnic minorities, particularly Black and Asian people, as well as groups such as travellers who have historically been underrepresented or misrepresented in health research
- Rural and coastal populations
- Groups with caring responsibilities
- Low health literacy, or those who speak English as a second language
- Younger populations, particularly people aged 18-24
Many of these groups share different potential barriers to getting involved with clinical research, shared by conference delegates as part of a wordcloud:

Where different communities have historically been excluded from taking part in clinical research, both awareness and trust of research and researchers might be low. Building on trust and on awareness are two closely related concepts, and are essential to being able to involve people in research.
If a researcher ‘parachutes’ in but doesn’t maintain a longstanding, trusting relationship, this might put up further barriers to collaborative and inclusive research. A decent segment of the posters presented at RDF25 focused on overcoming this at the individual study level – discussing examples of working closely with schools, mosques, farmers markets and GP surgeries in order to build up strong, long term relationships with historically underserved communities at regional levels.
There’s commitment to building trust with patients at so many different levels in the UK - the Health Research Authority announced at RDF25 that the theme of this year’s #MakeItPublic campaign for open and transparent will focus on sharing clinical trial results with patients.
Where are all the men?
We don’t traditionally think of men as an underserved community when it comes to health research.
But when four out of five of the people who die by suicide in the UK are men, and life expectancy is around four years shorter compared to women, Dr Tobit Emmens posed the question of why research participation in the UK, particularly for mental health, is so low for men.
This lack of research involvement is particularly exacerbated for younger men, as well as men from deprived regions, rural communities and ethnically diverse groups. When we introduce these intersectionalities, we can definitely consider these groups as underserved communities when it comes to clinical trials.
It’s hard to separate out why men might not participate in mental health research from why men might be reluctant to access mental healthcare, or any kind of healthcare, in the first place.
And so, we go back to that GP’s waiting room from my opening paragraph – why is it that those university-educated, white, middle-aged, middle class patients keen to get involved in research so often also happen to be women?
In a session on community engagement, Tobit Emmens suggested that maybe this is because men are taught from an early age within our culture to value stoicism, which conflicts with asking for help.
Is this a class issue too? Clinical trials don’t just conflict emotionally with being ‘tough’, but might also conflict logistically, with opening times and appointments clashing with inflexible working hours of jobs traditionally taken on by men.
Countering a reluctance to talk about mental health is tricky – and again relies on the importance of finding the right voices within strong, existing networks where trust can be built over time.

Boatyard Bikes in Exeter is perhaps the perfect example of investing in and building on existing community spaces built on trust. Their tagline is ‘we don’t just fix old bikes, we help fix people too’, reflecting their aim of providing an informal and non-clinical space for open discussions. The project builds on the idea that men usually prefer discussions side-by-side rather than face-to-face – perhaps giving us some important clues about what a ‘man-friendly’ clinical research space looks like?
Scaling Up
The challenge of how to build trust with historically underserved communities needs to be tackled at a national (and global) level to make research more equitable in the long term.
Clinical trials are not just about patients leading longer lives, but also about people spending more of their life in good health. There are huge disparities around this regionally across the UK – in some areas you can expect to spend 10 years longer in good health than in others. This figure varies not just by location but also by gender:
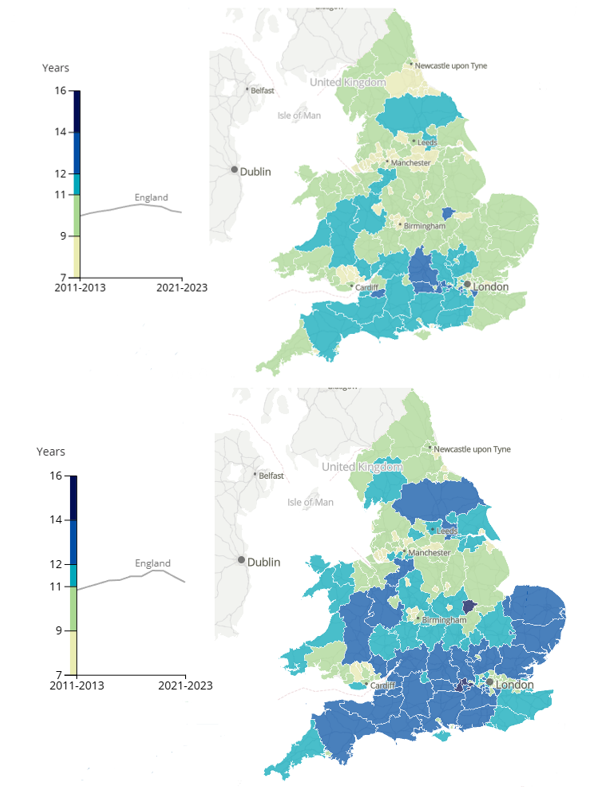
The disparity in long, healthy lives, and in clinical research participation is clearly being taken seriously in the UK. In 2022, the Be Part of Research service was launched by the NIHR on the NHS App, allowing the public to sign up for new and ongoing clinical studies across the UK.

An ongoing focus for Be Part of Research is considering how to address the inequalities in access to research which lead to underserved populations being less likely to get involved.
Be Part of Research also shared different researchers’ success stories of building ‘Research Ready Communities’ by finding local champions who were confident enough to have conversations within their communities, supporting others in getting involved with clinical research. A common thread in conversations was that while some communities might be hesitant to take part in research on new drugs and medicines, they might be more open to other types of research.
What are we doing at BMC and ISRCTN to build on this?
Patients’ trust in research goes hand in hand with transparency, echoed by the UK’s new regulations on clinical trials.
We’re proud that the UK’s Clinical Study Registry, ISRCTN, has close links with Be Part of Research. Our editorial team encourage and support Plain English Summaries of trial protocols, which we pass on to the BPOR site to help potential study participants understand when a trial is right for them, even if they don’t have a scientific background or English as a first language, minimising that barrier to participation.

ISRCTN’s editorial team at BMC have close links with the publishing team for Research Involvement and Engagement, keeping the inclusive design of trials and co-produced research at the forefront of our minds.
We’re also excited about how we can encourage researchers to think creatively about how they want to share their findings with participants and the public too – continuing to build that trust and understanding.
Follow the Topic
-
ISRCTN: The UK’s Clinical Study Registry

A primary clinical trial registry recognised by WHO and ICMJE that accepts studies involving human subjects or populations with outcome measures assessing effects on human health and well-being, including studies in healthcare, social care, education, workplace safety and economic development.
Your space to connect: The Primary immunodeficiency disorders Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine, Immunology, and Diseases!
Continue reading announcement




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
Great blog, Amy!
This is such a great read, Amy!