Engineering alcohol dehydrogenase for better utilization of nicotinamide cofactor biomimetics
Published in Bioengineering & Biotechnology, Chemistry, and Sustainability
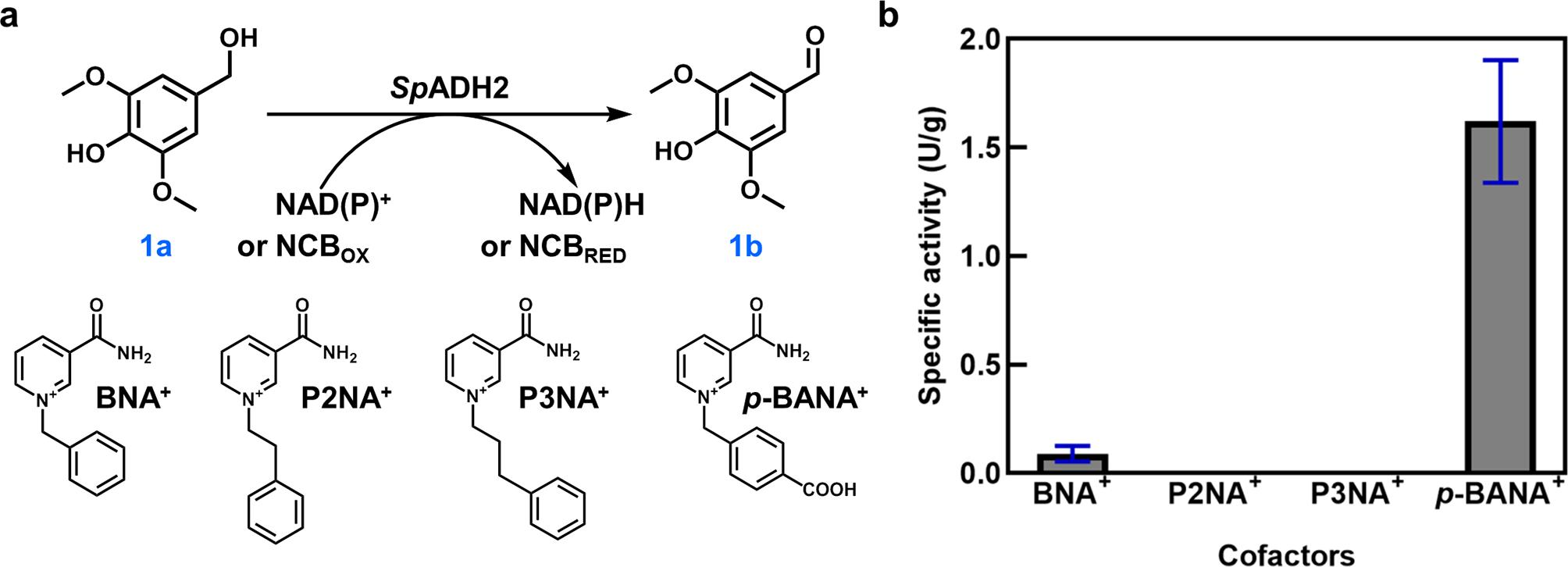
Nicotinamide cofactor biomimetics (NCBs) serve as low-cost alternatives to the expensive NAD(P)+/NAD(P)H, holding significant potential for applications in oxidoreductases. In this study, an alcohol dehydrogenase (SpADH2) from Sphingobium sp. SYK-6 was identified for the utilization of synthetic NCBs. SpADH2 exhibited a catalytic activity of 11.55 U/g in oxidation of syringyl alcohol when utilizing para-3-carbamoyl-1-(4-carboxybenzyl)pyridin-1- ium (p-BANA+) as cofactor. Semi-rational engineering of SpADH2 led to identification of key variants (H43L, A290I, H43L/A290I) with enhanced catalytic efficiency and specificity using p-BANA+ as the cofactor. Compared with wild-type, variant H43L/A290I exhibited a 7-fold increase in activity and an astonishing 6750-fold improvement in cofactor specificity ratio. Enzymatic characterization reveals that the substrate spectrum of SpADH2 could change significantly when utilizing different totally synthetic NCBs (tsNCBs). Furthermore, interaction analysis demonstrates critical roles of residues 43 and 290 in anchoring and release of p-BANA+. This study identified a natural ADH capable of utilizing totally synthetic NCBs, which has never been reported. Importantly, our results provide valuable ADH candidates for potential synthetic biology and industrial developments, and offer valuable guidance for identification and engineering ADHs toward utilizing NCBs as cofactors with improved catalytic performance.
Cite this article: Zhu Y., Zhou J., Gu X., Wang H., Han H., Ni Y. Engineering a newly identified alcohol dehydrogenase from Sphingobium sp. for efficient utilization of nicotinamide cofactors biomimetics. Bioresour. Bioprocess. 12, 41 (2025). https://doi.org/10.1186/s40643-025-00870-z
Follow the Topic
-
Bioresources and Bioprocessing

This journal aims at providing an international academic platform for exchanging views on and promoting research to support bioresource development, processing and utilization in a sustainable manner.
What are SDG Topics?
An introduction to Sustainable Development Goals (SDGs) Topics and their role in highlighting sustainable development research.
Continue reading announcement