Engineering Bifunctional Catalytic Microenvironments for Durable and High-Energy-Density Metal–Air Batteries
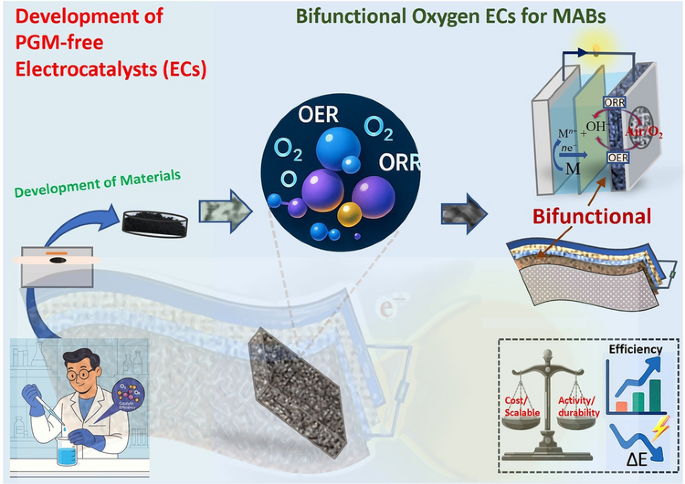
From grid-scale storage to foldable wearables, metal–air batteries (MABs) promise ultra-high energy at rock-bottom cost. Yet their commercial flight has been stuck on the runway: the air-breathing cathode must catalyse both the oxygen reduction (ORR) and evolution (OER) reactions, but almost all known materials excel at only one, succumbing to rapid degradation in the harsh redox micro-environment. Now, an international team led by Prof. Jean Marie Vianney Nsanzimana (University of Padova), Prof. Bao Yu Xia (Huazhong University of Science & Technology & Sungkyunkwan University), and Prof. Thandavarayan Maiyalagan (SRM Institute of Science and Technology) delivers a 360° roadmap for engineering bifunctional, noble-metal-free catalysts that can finally unlock durable, high-energy MABs across lithium-, zinc-, and sodium-air chemistries.
Why This Review Matters
- End-to-End Blueprint: For the first time, a unified framework links catalyst nano-/micro-environments, scalable synthesis, and full-cell validation.
- Universal Design Rules: Reveals how atomic coordination, vacancy engineering, and interfacial strain dictate bifunctional activity and stability.
- Scalable & Earth-Abundant: Focuses on low-cost transition-metal, carbon, and high-entropy systems ready for industrial scale-up.
Inside the Microenvironment Revolution
1. Single-Atom to High-Entropy Alloys
Single-Atom Catalysts (SACs)
- Fe–N4 & Co–N4 moieties anchored on nitrogen-doped carbon deliver ORR half-wave potentials above 0.85 V vs. RHE and OER overpotentials below 370 mV at 10 mA cm-2.
- Dual-atom Fe/I–N–C rods reach a power density of 197.9 mW cm-2 in quasi-solid ZABs, operating from −40 °C to 60 °C for over 280 h—tripling the lifetime of Pt/C–IrO2.
- Triple-atom Fe–Co–Ni–N6 sites modulate the d-band centre, pushing bifunctional ΔE (E_OER@10 – E_ORR1/2) to 0.61 V, a record among non-precious systems.
Spinel & Perovskite Oxides
- MnCo2O4/Mn2O3 nanorod heterostructures grown via one-step hydrothermal synthesis cut the ORR/OER gap to 1.16 V in full cells, outperforming Pt/C//IrO2 at high current densities.
- Mo-doped LaCoO3 (Co:Mo = 95:5) introduces precisely tuned Co3+/Co4+ redox couples and oxygen vacancies, yielding 136 mW cm-2 peak power and 120 h continuous cycling at 10 mA cm-2.
- Ruddlesden–Popper CeO2–PrSrNiCoO3 nanofibers form a hierarchical porous web that triples triple-phase boundary length; assembled ZABs deliver 161 mW cm-2 and survive 219 h with <5 % fade.
High-Entropy Alloys (HEAs)
- A fourteen-element HEA@oxide composite (PtPdAuAgCuIrRu HEA nanoclusters on AlNiCoFeCrMoTi)3O4 spinel) achieves ΔE = 0.61 V—lower than any reported earth-abundant system.
- Earth-abundant high-entropy spinel ferrites (MgCoCuNiZn)Fe2O4 retain the crystal field of noble-metal analogues while slashing raw-material cost by >90 %.
- Machine-learning-guided DFT screening of 729 dual-metal sites predicts RuCoN6, RuIrN6, OsRhN6, and OsCoN6 as next-gen champions, validating the micro-environment design philosophy.
2. Carbon & MOF-Derived Hybrids
Heteroatom-Doped Carbons
- B,N dual-doped porous carbon (BNPC-1100) derived from Zn-MOF pyrolysis exhibits ORR onset at 0.95 V and OER η = 0.34 V, powering coin ZABs for 100 h at 1.12 V discharge plateau.
- N,P-codoped hollow nanospheres templated by CsCl show a half-wave potential of 0.85 V and 0.34 V OER overpotential, rivalling Pt/C and IrO2 in both aqueous and solid-state cells.
- Se,N,B tri-doped biomass carbon (SeC900) from recycled paper cups delivers 1618 mAh g-1 in LABs with a 3.14 V open-circuit voltage.
MOF-Derived Composites
- Co3Fe7 alloy anchored on N-doped carbon (Co3Fe7–N–C-OAc) inherits the ZIF-8 pore hierarchy, doubling O2 flux and reaching 587 mW cm-2 in solid-state ZABs—best among non-precious systems.
- Confinement synthesis of Co@N–CNSs from Co-phthalocyanine@Zn-MOF yields 2–3 nm Co nanoparticles that survive 150 h at 10 mA cm-2 with 775 mAh g-1 specific capacity.
- One-pot pyrolysis of FePc@HCoNC generates atomically dispersed Fe–N4 and Co–N4 sites, achieving 758 mAh g-1 and 193 mW cm-2 in aqueous ZABs.
3. Operando Insights & AI-Driven Design
- In-situ X-ray absorption spectroscopy tracks the reversible Co2+/Co3+ redox shuttle in spinels during ORR/OER, correlating lattice-oxygen participation with activity.
- DFT + machine learning identifies the electronic descriptors (eg occupancy, Bader charge, oxygen vacancy formation energy) that predict ΔE with R2 > 0.91 across 700+ compositions.
- Multiscale modelling couples micro-kinetics with continuum transport, showing that hierarchical pores (>100 nm) reduce concentration overpotential by 40 % at 20 mA cm-2.
Toward Market-Ready MABs
- Wearable Power: Quasi-solid ZABs with Fe–N–C cathodes retain 85 % capacity after 1,000 bending cycles at a 5 mm radius—ideal for e-textiles and epidermal sensors.
- Cold-Climate Grids: Flexible LABs using Se-doped carbon cathodes deliver 1,618 mAh g-1 at −40 °C, paving the way for polar research stations and aerospace micro-satellites.
- Containerised Storage: A 1 kWh Zn–air module with MnCo2O4/Mn2O3 cathodes cycles 2,300 times with <10 % energy fade—competitive with LFP systems at one-third the cost.
Next Steps to Gigawatt Deployment
- Roll-to-Roll Synthesis: Adapt ALD and flash-Joule heating for metre-scale SAC and HEA foils without noble metals.
- AI-Guided QA/QC: Embed impedance spectroscopy and Raman probes in coating lines for real-time defect detection.
- Pilot MWh Stacks: Commission 100 kWh demonstrators integrating fire-retardant hydrogel electrolytes and 3D-printed air electrodes by 2027.
Stay tuned as these micro-environment design rules migrate from coin cells to containerised storage and space-qualified packs—making MABs the true everywhere, every-weather battery.
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in