
Despite their diminutive size, microscopic algae, also called phytoplankton, play a massive role in the global ocean. Aside from providing the foundation for the marine food web, phytoplankton are responsible for roughly half of the Earth’s annual primary production, making them centrally important to global carbon cycling and the oxygen we breathe.
Historically, phytoplankton have been assumed to be autotrophic, producing carbon biomass using energy from the sun via photosynthesis. However, over the last several decades, it has become clear that phytoplankton often supplement autotrophic photosynthesis with the ingestion of other organisms via phagocytosis—a combination of strategies referred to as phago-mixotrophy. The extent to which phytoplankton feed mixotrophically can have big implications for marine ecosystems.
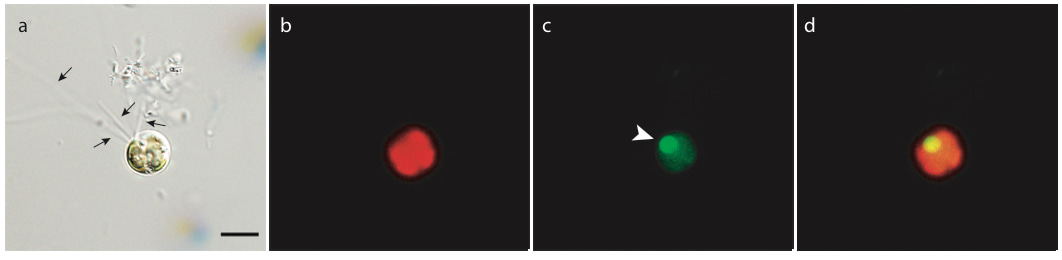
Despite the outsized importance of phago-mixotrophy, it can be difficult to capture. In our ISMEJ article “Experimental Identification and in silico Prediction of Bacterivory in Green Algae” we report results from a series of feeding experiments where we inoculated algal cultures with fluorescently labeled bacteria (FLB), and monitored the algal population for the ingestion of these prey using flow cytometry and epifluorescent microscopy.
One challenge, though, is that algal cells can be enormously picky eaters. Just finding a palatable proxy prey was difficult, with our strains apparently favoring live prey over dead or inert particles . Algal appetites can also be modulated by irradiance, concentrations of dissolved nutrients, and the culture growth stage. Getting reproducible results meant getting to know the feeding preferences of each algal strain over numerous iterations of culture growth and experimentation.
Each feeding experiment required growing the respective algae strains in culture flasks for almost two weeks (figure 1). The bacterial prey also had to be grown in a separate flask, before reaching sufficient densities for the labeling protocol. Only once our algal strains were grown to a bright green tinge and our bacteria were fluorescently labeled, could we proceed to the feeding experiments.

The experiments themselves were complicated by the fact that the cytometer and the microscope were located 20 miles apart: the microscope in Dr. Eunsoo Kim’s lab at the American Museum of Natural History on New York City’s Upper West Side, and the cytometer in Dr. Solange Duhamel’s lab at Columbia University’s Lamont-Doherty Earth Observatory—10 miles north of the city. It was a collaborative effort: In order for the FLB to be in two places at once, we devised a plan of early morning “FLB handoffs”, with a delicious air of covert clandestinity, at the LDEO shuttle stop before Nick rode off to start the flow cytometry and Sophie headed to the AMNH for the microscopy.
In all, we were able to confirm feeding in five strains of green algae (banner image, figure 2), highlighting important sources of methodological bias that could contribute to underestimates of mixotrophic feeding and corroborating the predictions of the gene-based model of Burns et al. (2018). Our results demonstrate the potential for widespread mixotrophy across green algae, and also set the stage for future experimental work to further illuminate the importance of algal phago-mixotrophy at global scales.
References
Burns J.A., Pittis A.A., & Kim E (2018). Gene-based predictive models of trophic modes suggest Asgard archaea are not phagocytotic. Nature Ecology & Evolution, 2, 697–704.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in