Feeding the enemy: Using Ivermectin spiked blood as a mosquito control strategy
Published in Microbiology and Zoology & Veterinary Science

Malaria is a public health concern worldwide, despite being both preventable and curable. Vector control strategies like insecticide-treated bed nets and indoor residual spraying have proved effective against mosquito species that typically bite indoors at night, however mosquito species have diversity in their feeding behaviours which needs to be considered if we want to effectively reduce malaria incidence worldwide. For example, some species bite outdoors rather than indoors, during the day instead of the night, and others prefer biting animals over humans. These species have little contact with things like insecticide-treated nets, so alternative control strategies need to be developed that target these species.
Research has shown that treatment of a vertebrate host with a systemic insecticide (endectocide) that a vector is then exposed to via their blood meal is an effective way to reduce vectors of disease. For example, this method has been seen to reduce sand fly vectors of leishmaniasis and flea vectors of plague. In the past few years, the method has emerged as a potential vector control that could help eliminate malaria, with many studies analysing its effectiveness at reducing mosquito survival. For example, introducing ivermectin to cows was shown to reduce the survival of the cattle feeding mosquito species Anopheles arabiensis. Kobylinski et al set out to find the optimum methodology for endectocide and ectocide malaria control to standardise this strategy. The team undertook several experiments using Anopheles dirus and Anopheles minimus to assess things like ivermectin uptake and mosquito mortality. Powdered ivermectin was dissolved in dimethylsulfoxide and serial dilutions were made with human AB+ plasma. Mosquitoes were then put into a carton with a glass membrane feeder on top that contained blood or plasma ivermectin meals.
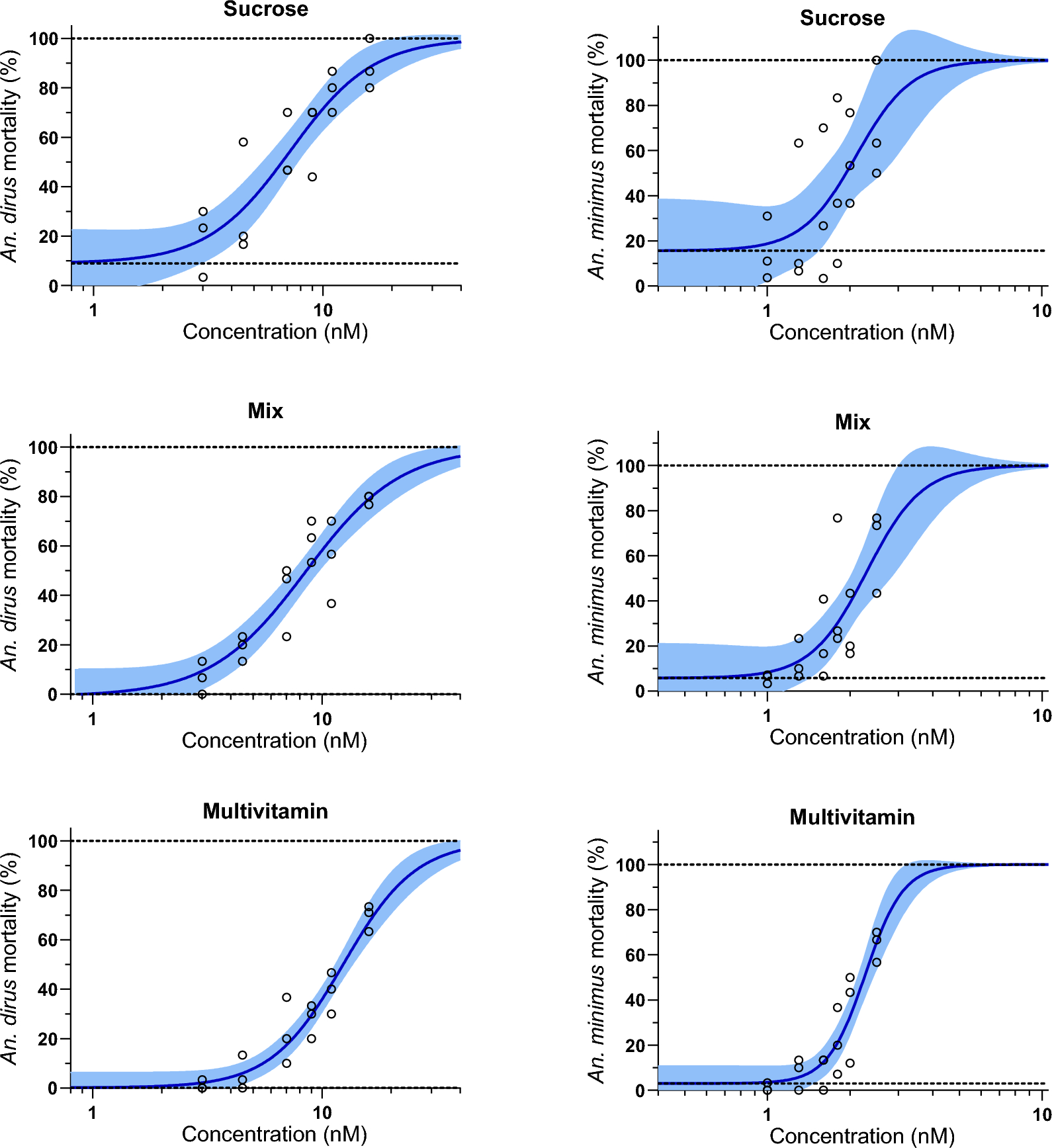
The first experiment split the mosquitoes into three groups which were fed three different sugar diets: ‘sucrose’- 10% sucrose up until the experimental membrane feeding began; ‘mix’- two days of a 5% multivitamin syrup with 5% sucrose, and then 10% sucrose until the experiment began; and ‘multivitamin’- 5% multivitamin syrup with 5% sucrose up until the experiment began. In Anopheles dirus the lethal concentration that killed 50% (LC50) was the same for both the ‘sucrose’ and ‘mix’ diet but was a lot higher for the ‘multivitamin’ diet, whereas in Anopheles minimus the L50 was the same for all diets.
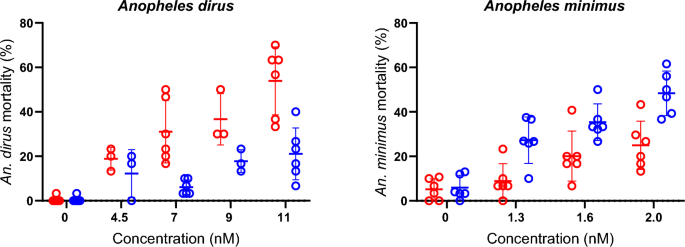
The second experiment looked at the difference in impact of ingesting ivermectin in either fresh plasma, fresh blood, or frozen blood on mosquito survival. The meal type effected the mosquito’s likelihood to feed, for example there was a substantial decrease in feeding when offered previously frozen blood, with nearly no feeding from An. minimus. However, there was no substantial difference in the likelihood of feeding on the other two meal types. Surprisingly, the impact of the meal type on mortality was the opposite in both species, with ivermectin being significantly more lethal in An. dirus when ingested in a blood meal compared to a plasma meal (at all but one concentration of ivermectin). Conversely, ivermectin was significantly more lethal to An. minimus when ingested in plasma than in blood at all concentrations.
The final experiment set about quantifying ivermectin concentrations from blood meals compared to the amount making it into the midguts of mosquitoes. After dissecting mosquitoes of each species, Kobylinski et al reported a 20.3% and 23.4% reduction in ivermectin concentration in the midgut compared to the blood meal in An. dirus and An. minimus respectively, showing no substantial difference between both species.
The outcome of the team’s research led them to come up with several methodological suggestions. Firstly, they point out that a known concern when mixing certain human blood groups is the resultant red blood cell lysis (haemolysis). To combat this, they suggest that plasma from AB+ people be used for endectocide and ectocide dilutions as haemolysis does not occur with this blood type. As haemolysis does not occur in cattle this is not a concern for this species, however further evaluation may be required for other species. As the ivermectin plasma and blood meals had opposite lethality's on the two different species, the authors stress the need to reconstitute the plasma with red blood cells to achieve similar outcomes to that of the whole blood. The authors also point out that their use of human plasma and amber glass vials for the preparation of ivermectin dilution led to a final concentration of the blood meals that was close to the target concentrations. This is compared to previous studies that have used PBS dilution and plastic centrifuge tubes that have resulted in a 20-80% reduction from the intended concentrations. Based on their sugar diet experiments they also recommend the ‘Mix’ sugar diet for 48-hours post emergence followed by 10% sucrose until the experiment begins.
Kobylinski et al’s study contributes to the literature available on using endectocide or ectocide for control of mosquito species that are not frequently exposed to current effective control methods like insecticide-treated bed nets and indoor residual spraying. Their study offers some useful methodological suggestions for using ivermectin as a mosquito control method and proposes several areas of further research that are needed to ensure the process can be standardised and undertaken in the most effective way possible.
Follow the Topic
-
Parasites & Vectors

This journal publishes articles on the biology of parasites, parasitic diseases, intermediate hosts, vectors and vector-borne pathogens.
-
BugBitten

A blog for the parasitology and vector biology community.
Related Collections
With Collections, you can get published faster and increase your visibility.
Credelio Quattro
Articles published in the collection have already gone through the systematic peer review process of the journal.
Publishing Model: Open Access
Deadline: Apr 24, 2026
Molecular biology of disease vectors
Parasites & Vectors is calling for submissions to our Collection on 'Molecular biology of disease vectors.' Vector-borne diseases such as Chagas disease, dengue and chikungunya, human African trypanosomiasis, leishmaniasis, lymphatic filariasis, onchocerciasis and schistosomiasis, are among the WHO neglected tropical diseases, and associated with devastating health, social and economic consequences. Studying various aspects of disease vectors is a fundamental step towards the control of these diseases. This collection contains articles from keynote speakers presenting at the 10th Workshop on Genetics and Molecular Biology of Insect Vectors of Tropical Diseases (Entomol10), held during the 59th Congress of the Brazilian Society of Tropical Medicine, São Paulo, Brazil.
The collection is also open to submissions on molecular biology (e.g., population genetics, phylogenetics and evolution, molecular taxonomy, and vector-pathogen interactions), surveillance and control of disease vectors.
Publishing Model: Open Access
Deadline: Feb 28, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in