
In my early days at the University of Alabama at Birmingham, the concept of a lung microbiome was still in its infancy. The Human Microbiome Project hadn’t included the lungs as a target tissue for study, setting back our understanding of its microbial dynamics a good 10 years behind that of the gut and the skin.
The birthplace of our research was, fittingly, the Neonatology Department at UAB. As a neonatologist, my research focused on understanding the mechanisms underlying airway diseases of premature infancy like Bronchopulmonary Dysplasia (BPD). At the time, we had recently shown that infants had a lung microbiome at birth, challenging the previously held thinking that the airways were sterile. We analyzed premature infants’ tracheal aspirate samples across multiple cohorts, using the microbial signatures to predict whether or not the patient would develop BPD. Accordingly, we found that an enrichment of proteobacteria and depletion of firmicutes was congruent with a BPD phenotype, while the opposite was true in resisting its development.
Around the same time, I started collaborating with Dr. Amit Gaggar in the Department of Pulmonary, Allergy, and Critical Care Medicine, an expert in protease biology. His research had defined a role for matrikines, bioactive fragments of the extracellular matrix (ECM), in driving airway tissue degradation in pulmonary disease. Acetylated PGP (Ac-PGP) in particular is generated when an enzyme called matrix metalloproteinase 9 (MMP-9) degrades collagen in the ECM, liberating Ac-PGP to trigger a swath of pro-neutrophilic inflammatory cytokines downstream in an attempt to signal for tissue repair.
In order to connect the dots between the proteobacteria phenomenon we had observed in patients and the Ac-PGP/MMP-9 mechanism we had observed in Dr. Gaggar’s lab, we generated multiple murine models. We found that dual exposure to hyperoxia and E. coli-derived lipopolysaccharide (LPS) in mouse pups increased tissue damage, decreased lung function, and increased biomarkers of neutrophilic inflammation. Overexpression of Ac-PGP function exacerbated the damage while inhibition attenuated it.
We had worked from the bedside to the bench to understand the mechanisms at work; the next step was to go back from the bench to the bedside to consider a therapeutic intervention. Our hypothesis was rooted in our earlier findings: what if we could supplement firmicutes to counteract the neutrophilic inflammation and subsequent airway damage?
We returned to our murine models of BPD and did exactly that, dosing mouse pups with a blend of live Lactobacilli strains intranasally. We observed significant improvements across tissue structure and biomarkers in the lungs. This got us more curious about 1. the underlying mechanisms to the commensal bacteria’s anti-neutrophilic activity and 2. our approach’s applicability in adult airway disease.
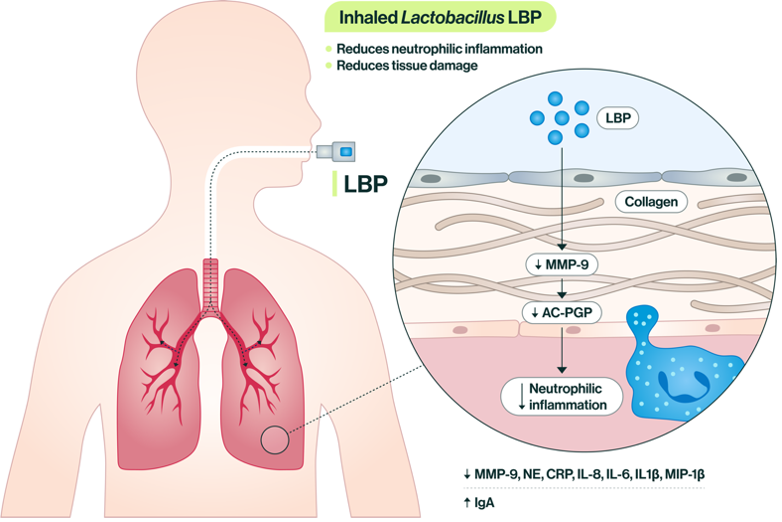
Figure 1. Inhalation of a live Lactobacilli strains has a number of downstream effects largely related to the reduction of neutrophilic inflammation in airway tissue.
Through a series of in vitro and in vivo tests, we identified L(+) lactic acid as a marker of activity for the Lactobacilli that was at least in part responsible for the reductions in neutrophilic inflammatory pathway activity we had observed. When mice were administered the Lactobacilli blend to the lungs, the strains were cleared by the lungs over time, but the metabolic signature of L(+) lactic acid remained active for longer.
We turned our attention to making this potent blend of commensal bacteria more readily inhalable. In partnership with many of the scientists involved in developing the first inhaled insulin formulation, we used particle engineering to spray dry the bacteria into a flowable powder while, importantly, maintaining the viability of strains. Thus, an inhaled live biotherapeutic (LBP) was born.
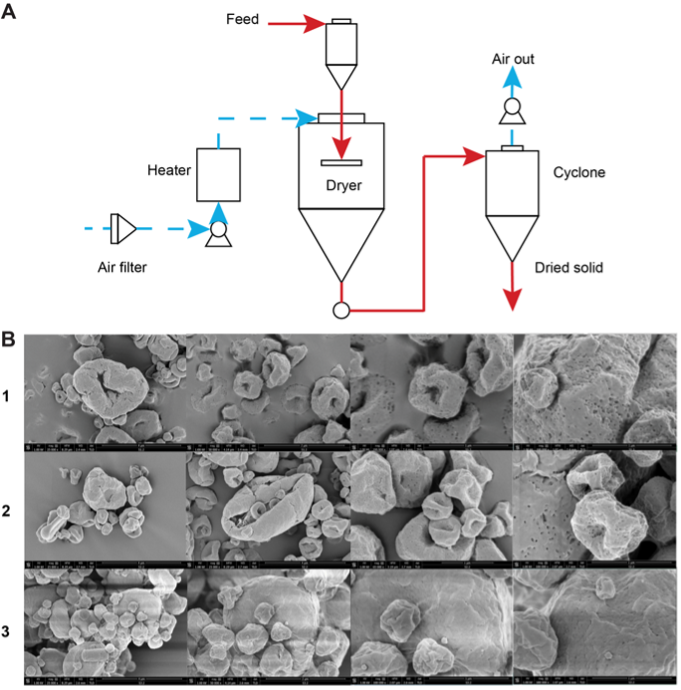
Figure 2. Top: flow chart of spray drying process used to particle engineer live Lactobacilli. Bottom: Scanning electron microscope (SEM) images of powder formulations.
Armed with an active drug product powder, we tested the Lactobacilli blend extensively in adult mouse models of Chronic Obstructive Pulmonary Disease (COPD). We challenged mice with porcine pancreatic elastase (PPE) and E. coli-derived lipopolysaccharide (LPS), our own twist on modeling severe, exacerbated COPD. In pre- and therapeutic treatment schemes, we observed major improvements in lung tissue structure as well as biomarker improvements in the serum, lung tissue, and bronchoalveolar lavage.
To pressure test the safety of the Lactobacilli LBP, we conducted respiratory safety and biodistribution studies in PPE-exposed mice to model inhalation in a disease state. In addition to 100% survival of the mice tested, all vital signs remained within normal ranges. Of note, the Lactobacilli strains did not distribute beyond the lungs into the serum, brain, heart, liver, or kidneys, nor did it engraft in the lungs. These were positive indications that inhalation of the live bacteria could be suitably safe for delivery.
On a journey of over 10 years of exploration, the heart of it all has remained patients’ lives. By pioneering inhaled LBPs as a new class of drugs, we push towards a future where patients have a wealth of treatment options at their fingertips. A future where steroids aren’t the only option and side effects with recurrent use are minimized. The human microbiome is a vast resource that we’ve only just begun to explore – who knows where it will take us next?



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in